How many atoms are in this formula?
H2O
Three atoms:
Hydrogen = 2
Oxygen = 1
What is on the periodic table?
Elements
Which is an example of a molecule? C2, He, NH3, Br, H2O
C2
Which is not a compound? CO, H2, NH3, CH4
H2
What are pure substances that are not able to be broken down chemically called?
How many atoms are in this formula?
Na2
2 atoms
Na = 2
C, H, N, and O stand for what elements?
Carbon, Hydrogen, Nitrogen, and Oxygen
This molecule, made up of two hydrogen atoms and one oxygen atom, is essential for all life on Earth.
(10 Bonus Points if you tell me what it makes)
H2O=Water
How many compounds are in this formula?
CO2 + H2O -> C6H12O6 + O2
Three compounds
CO2, H2O, C6H12O6
O2 is a molecule
What makes up elements?
Atoms
How many atoms are in this formula: H2SO4
7 atoms.
Hydrogen = 2
Sulfur = 1
Oxygen = 4
How many elements are in this formula: C7H5(NO2)3
4
This simple molecule consists of one carbon atom and two oxygen atoms and is a major contributor to the greenhouse effect.
(10 Bonus Points if you tell me what it is)
CO2=Carbon dioxide
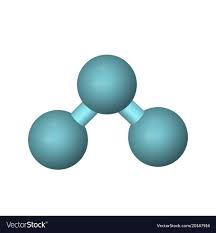 Is this a compound?
Is this a compound?
No, because all atoms are the same.
Two or more atoms of different elements that are chemically combined is called a
Compound
How many atoms are in this formula: C4H10
14 atoms
How many elements are in this formula?
Ca3(PO4)2
3
True or False a molecule can be a compound?
True
Is this molecule a compound?
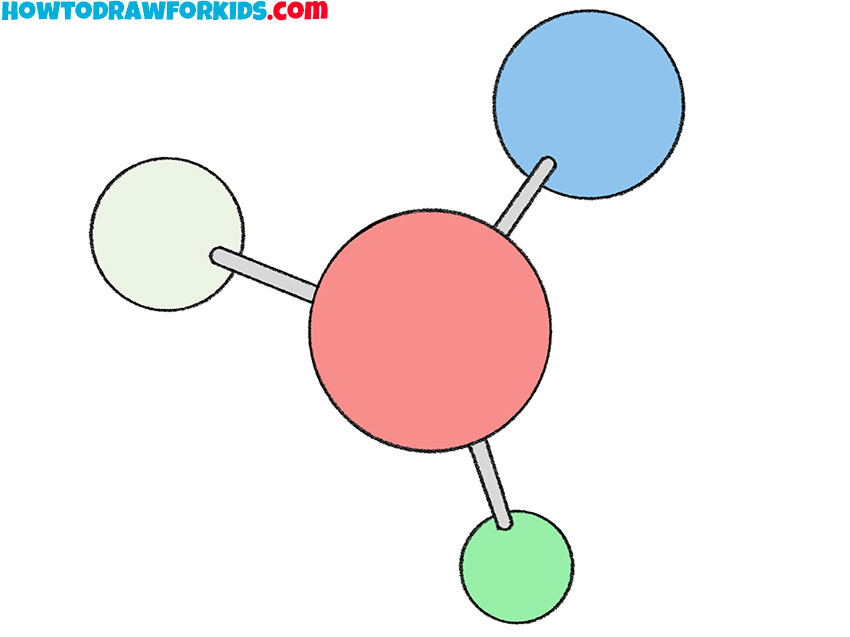
Yes
Two or more of the same atoms chemically combined is called a
Molecule
How many atoms are in this formula: 2CaH2PO4
16 atoms
Ca=2, H=4, P=2, O=8
How many atoms and how many elements are in this formula?
HgBr2
3 atoms, 2 elements.
What is the difference in a compound and a molecule?
How many compounds are in this formula?
CH4 + Cl2 --> CCl4 + HCl
Three compounds
True/False:
Compounds cannot be chemically broken down, while elements can.