TABLE
What is the atomic symbol?
H

What element is this?
Carbon (Look carefully - there's 6 protons)
How does static electricity work? (what part of an atom moves?)
Electrons move from one material to another. (ONLY THE ELECTONS - protons or neutrons NEVER move!)
A cell is smaller than an atom, and an electron is smaller than a proton.
FALSE
 What's the atomic mass?
What's the atomic mass?
12.0107
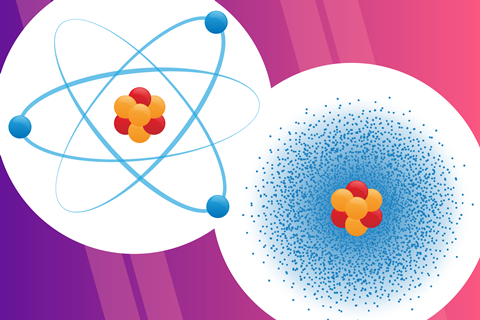
Which model more correctly shows what electrons are really doing in an atom?
the one on the right (electrons form a cloud as they orbit, like in Schrodinger's model in 1926)
Atoms are made up of what?
Protons, Electrons and sometimes neutrons
What part of an atom is free to move around from one atom to another?
Pure gold is made up of 3 kinds of atoms.
False (It's only made up of gold ATOMS, not molecules or other atoms)
 How many neutrons are in here?
How many neutrons are in here?
44
Which element is HELIUM?
B
How do you find the number of neutrons?
Number of neutrons=atomic (mass rounded)-atomic number
What's the atomic mass of CRAZIUM, a fictional element with 200 protons, 300 neutrons, and 400 electrons?
500
molecules are made when atoms are combined.
true
How many protons are in Fluorine?
9
What do electrons repel? (use your notes from last-time if you need to!)
negative charges, or other electrons
 Which of these is a MOLECULE?
Which of these is a MOLECULE?
All but the lonely Helium
Are two atoms of the same element identical?
yes. Two atoms of the same chemical element are identical.
What element is this?
Lithium (3 protons)
 How many electrons does this have?
How many electrons does this have?
117
What do you call something with more than one atom?
molecule

Which of these atomic models correctly expanded the understanding of a nucleus having two different particles - the proton and neutron?
Bohr's model in 1913
Name at least 3 things wrong with this picture
Needs a nucleus, needs neutrons, needs protons, electrons need to be orbiting the middle
True
How many electrons are in OXYGEN?
8