The positive particles found in the center of an atom are called: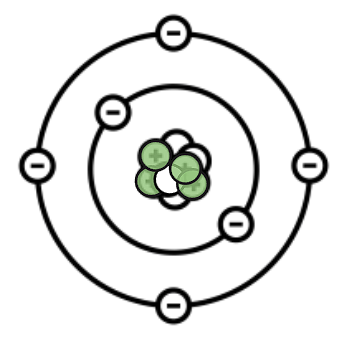
Protons
What is this element's symbol?
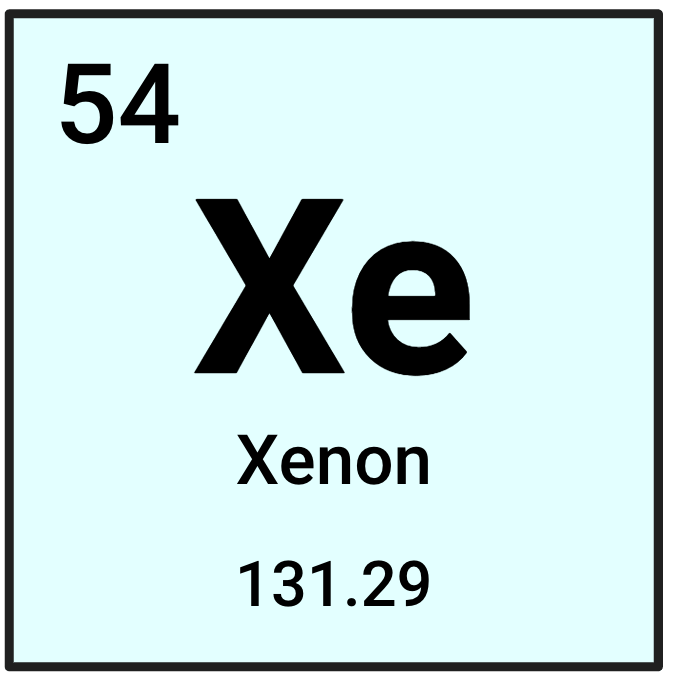
Xe
How many electrons can the 1st shell of an atom hold?
2
How do you calculate how many neutrons an atom has?
Round the atomic mass, then subtract the atomic number.
Which element family does Silicon (Si) belong to?
Metalloids

The negative particles found in the space surrounding the center of an atom are called:
Electrons
What is this element's Atomic Number?
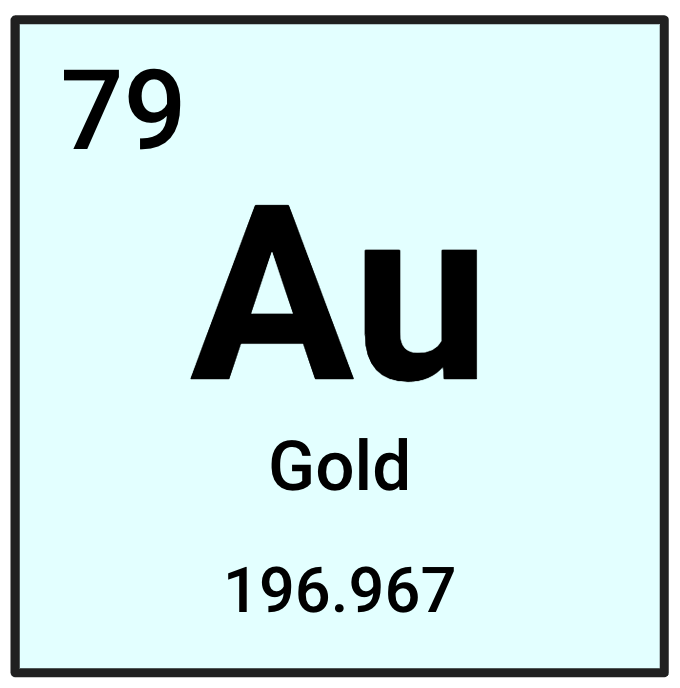
79
How many electrons can the 2nd shell of an atom hold?
8
Which element does this Bohr Diagram belong to?
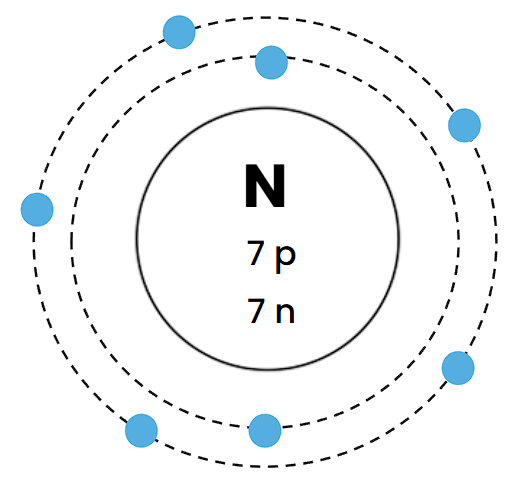
Nitrogen

Which element family does Chlorine (Cl) belong to?

Nonmetals

The neutral (no charge) particles found in the center of an atom are called:
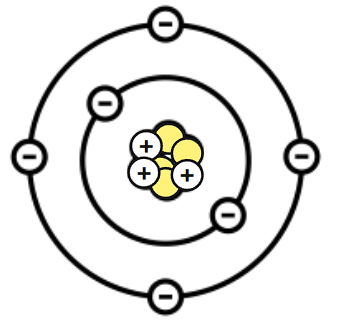
Neutrons
What is this element's Atomic Mass?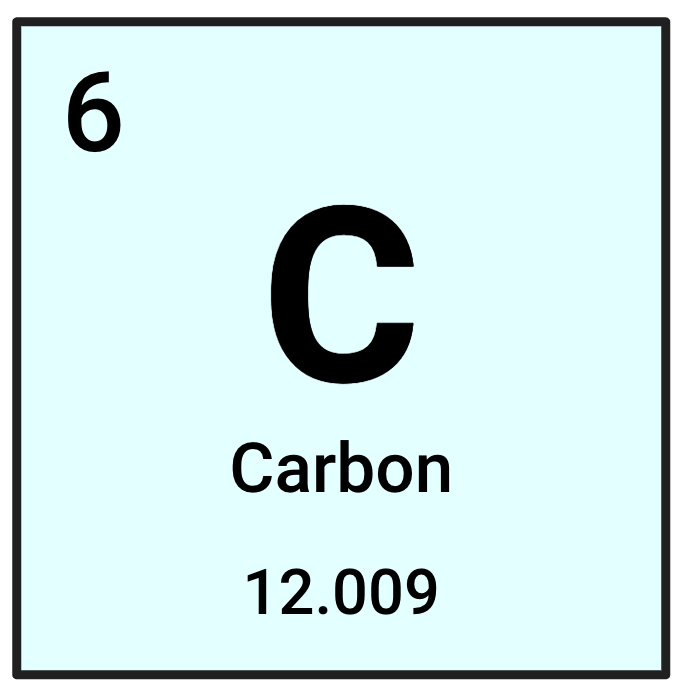
12.009
How many electrons can the 3rd shell of an atom hold?
8
Which element does this Bohr Diagram belong to? (The symbol has been hidden.)
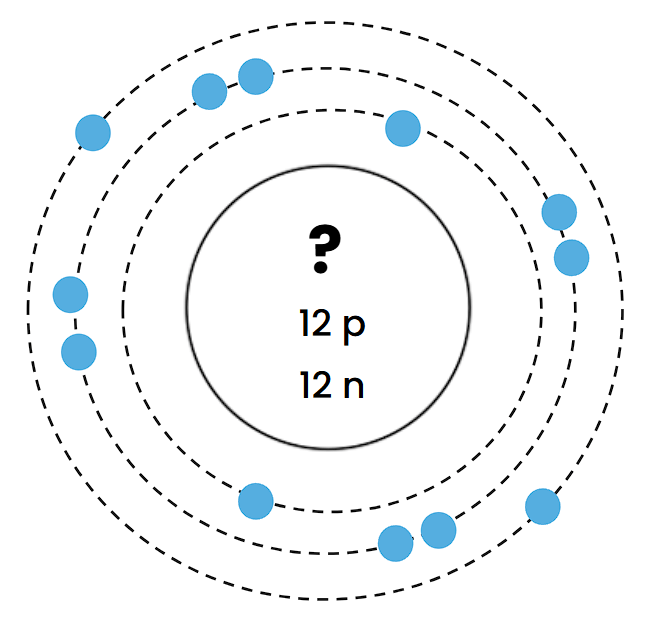
Magnesium (Mg)

Which element family does Radium (Ra) belong to?

Metals

The center region of an atom that holds protons and neutrons together is called the:
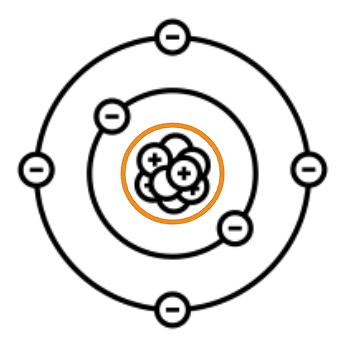
Nucleus
How many protons (+) and electrons (-) does a neutral Fluorine atom have?
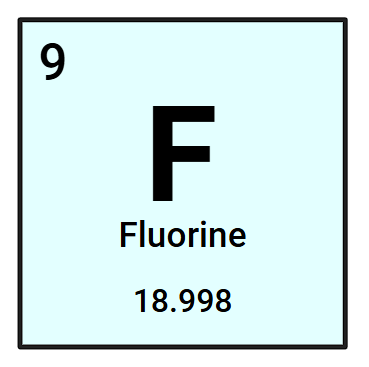
9 protons and electrons
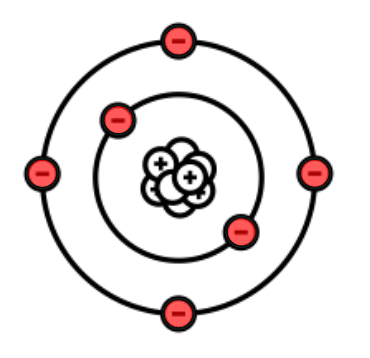
How many electron shells does this atom have?
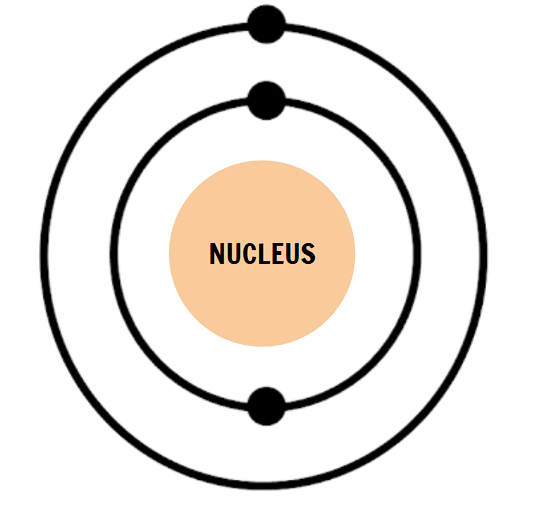
2

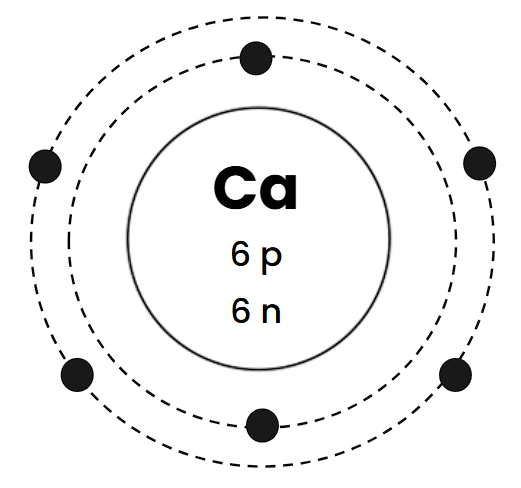
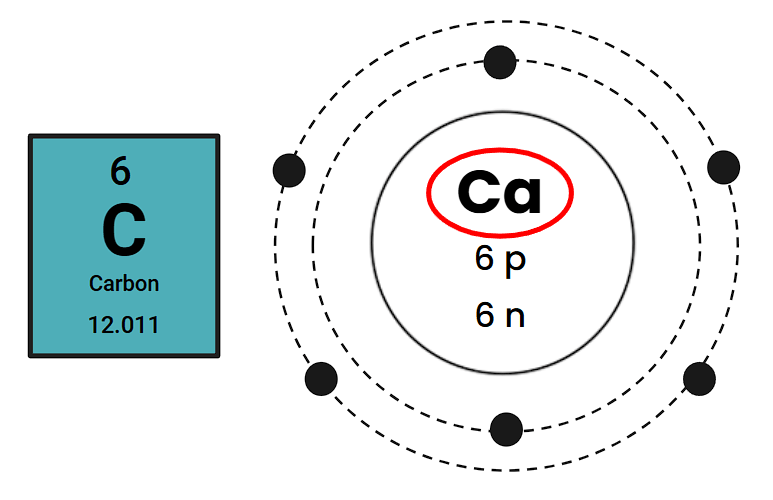
What is wrong with this Bohr Diagram?
Each period (rows) represents the number of:
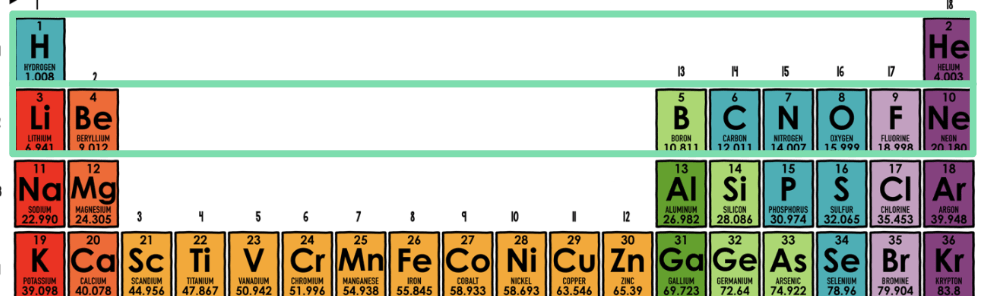
Electron Shells
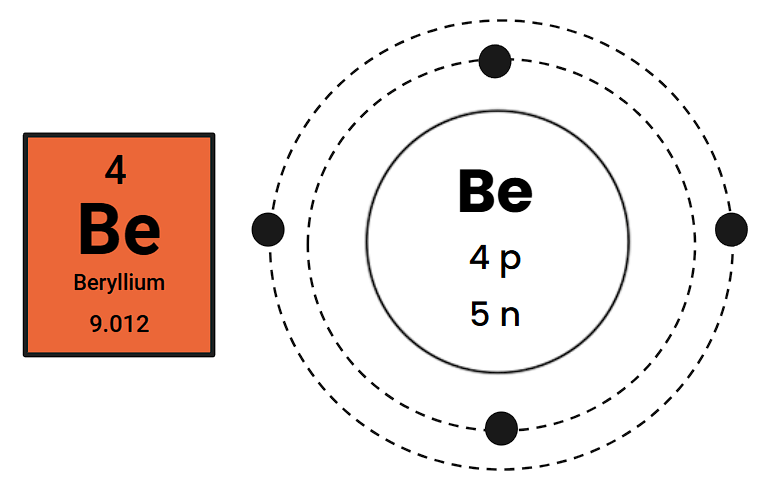
How many protons, electrons, and neutrons does this atom have?
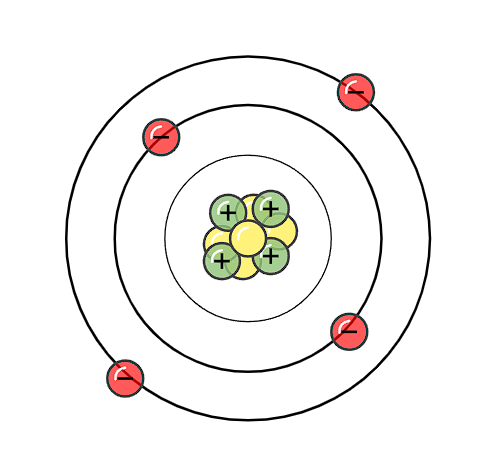
Protons: 4
Neutrons: 5
Electrons: 4
Locate the element Lead on your Periodic Table. Write it's:
Atomic Number, Symbol and Atomic Mass
Atomic Number: 82
Symbol: Pb
Atomic Mass: 207.2

How many electron shells does this atom have?
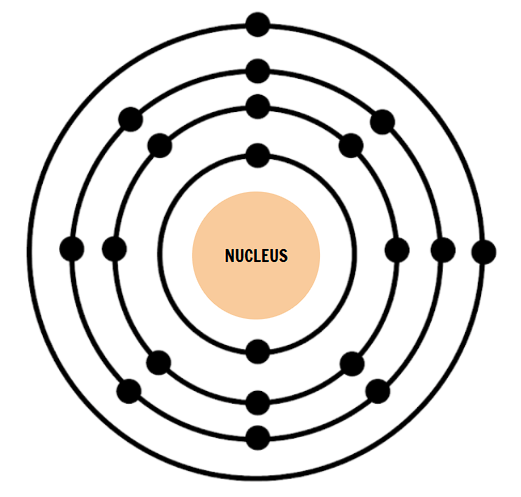
4

Draw a Bohr Diagram for the element Beryllium:

Each group (columns) represents the number of:
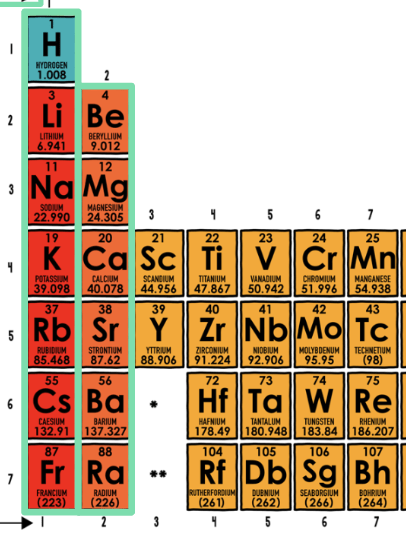
Valence Electrons