The positive particles found in the center of an atom are called: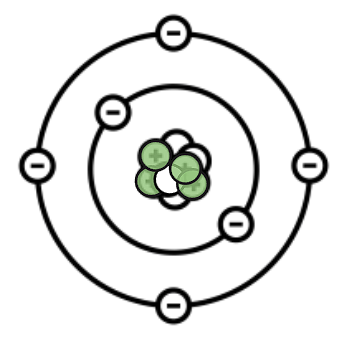
Protons
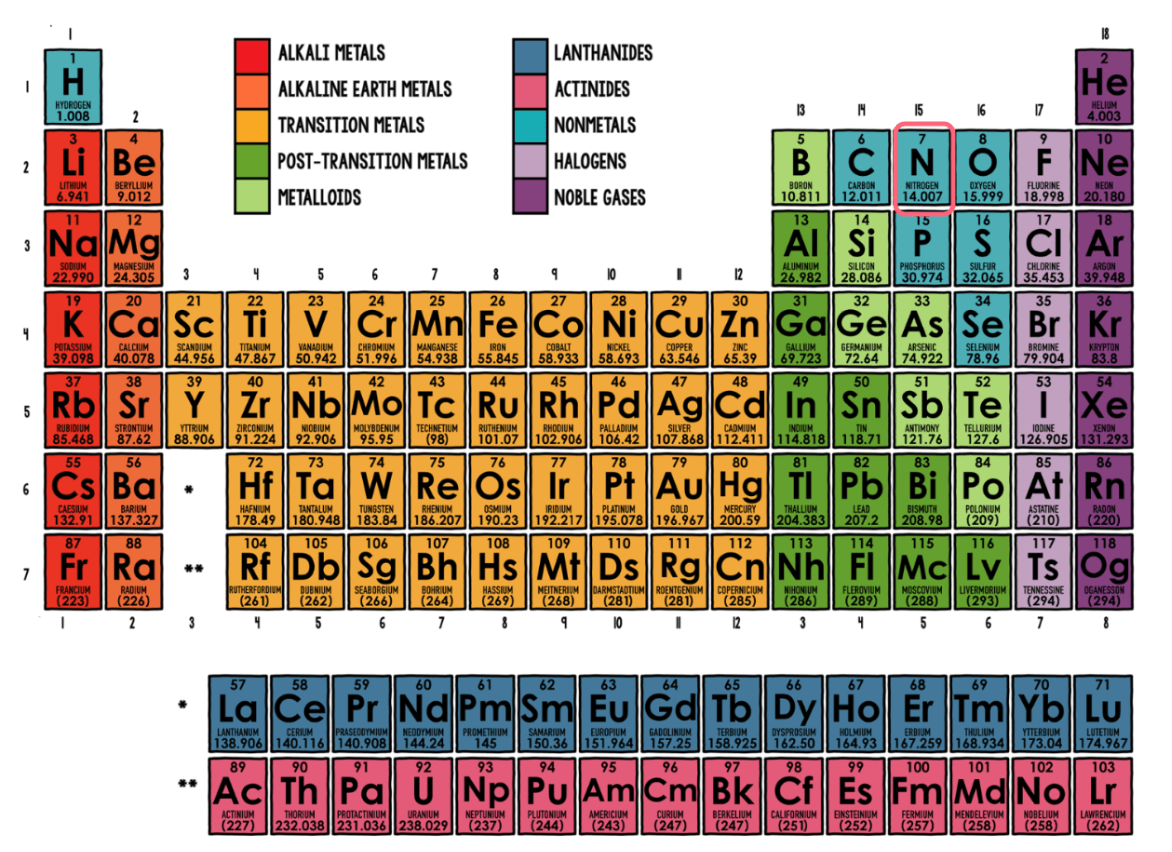
What is this element's symbol?
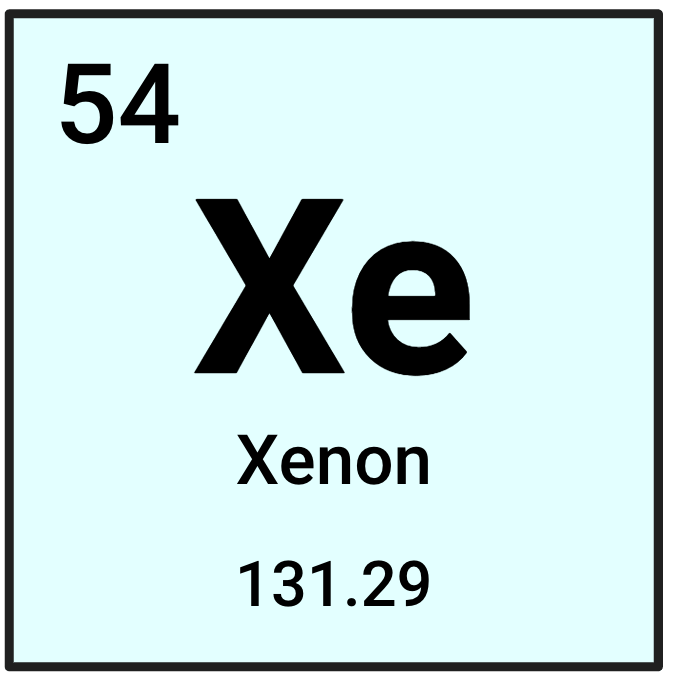
Xe
How many electrons can the 1st shell of an atom hold?
2
What are "valence electrons"?
Electrons in the outermost (outside) shell
Fill-in-the-blank: Elements are listed in order by Atomic __________.
Atomic Number OR Atomic Mass (both answers are correct)
The negative particles found in the space surrounding the center of an atom are called:
Electrons
What is this element's Atomic Number?
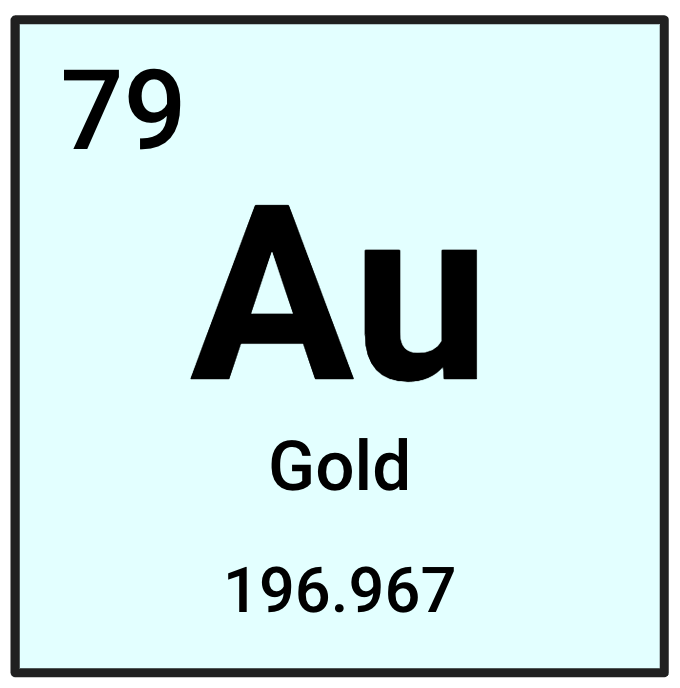
79
How many electrons can the 2nd shell of an atom hold?
8
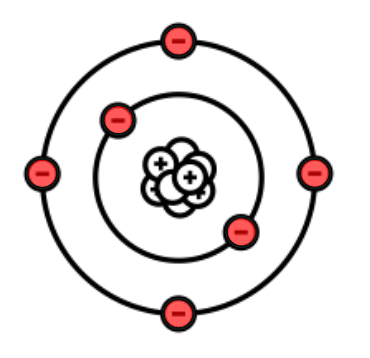
How many valence electrons does this element have?
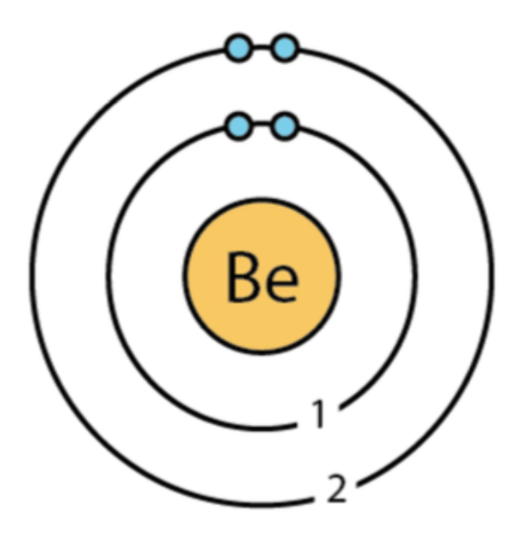
2
Which element family does Nitrogen (N) belong to?
Nonmetals
The neutral (no charge) particles found in the center of an atom are called:
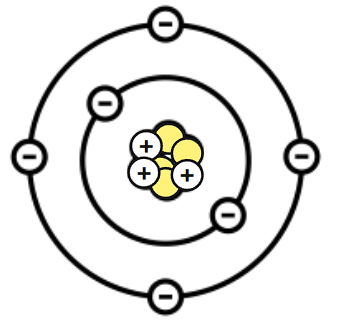
Neutrons
What is this element's Atomic Mass?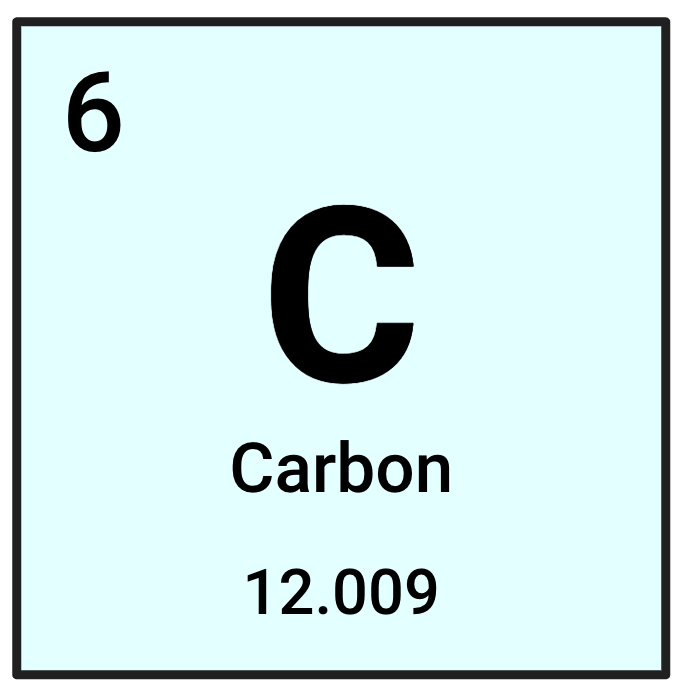
12.009
How many electrons can the 3rd shell of an atom hold?
18
How many valence electrons does this element have?
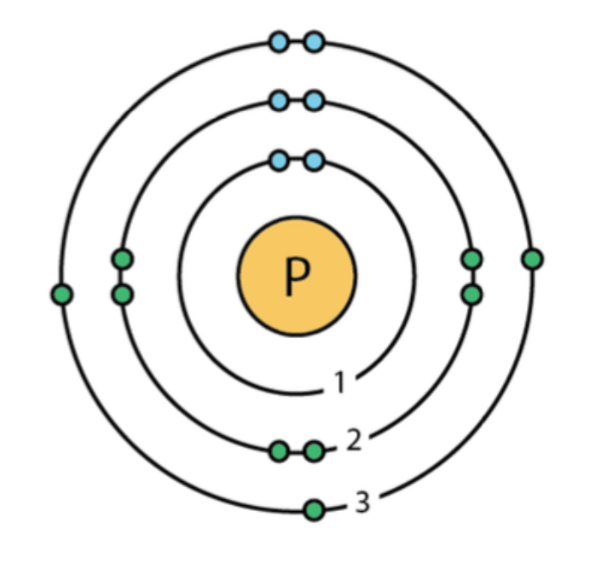
5
Which element family does Radium (Ra) belong to?
Alkaline Earth Metals
The center region of an atom that holds protons and neutrons together is called the:
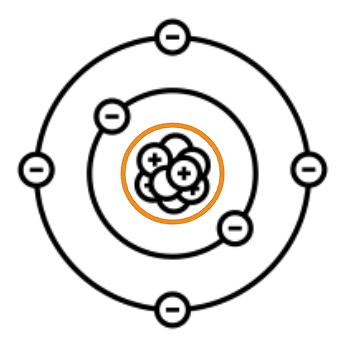
Nucleus
How many protons (+) and electrons (-) does a Fluorine atom have?
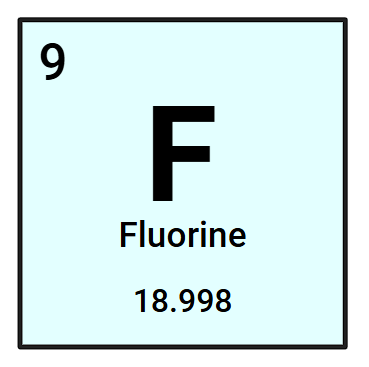
9 protons and electrons
How many electrons can the 4th shell of an atom hold?
32
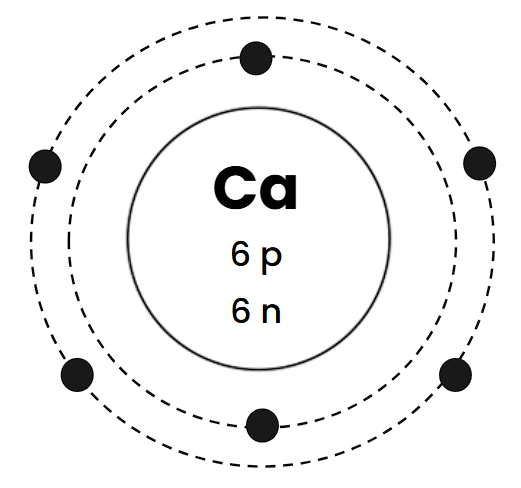
What is wrong with this Bohr Diagram?
Each period (rows) represents the number of:
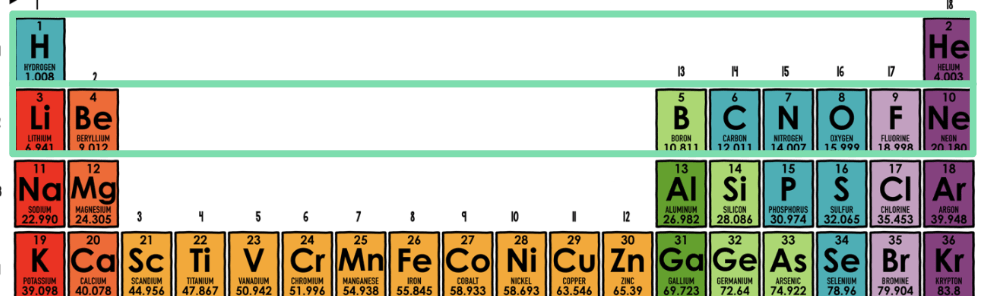
Electron Shells
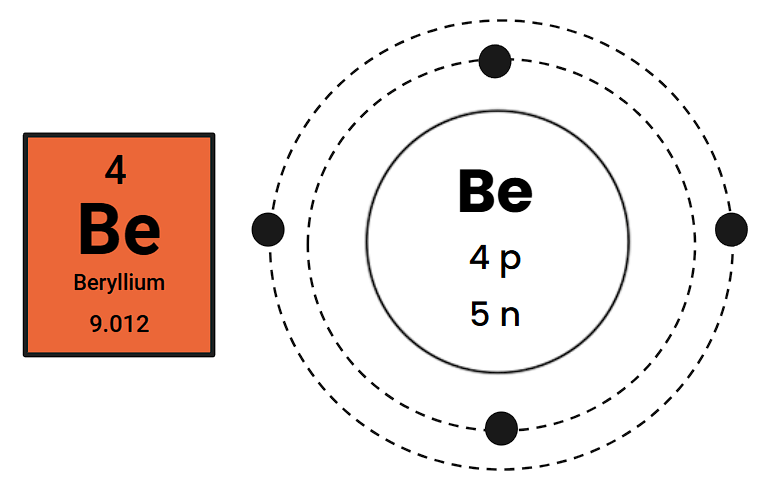
How many protons, electrons, and neutrons does this atom have?
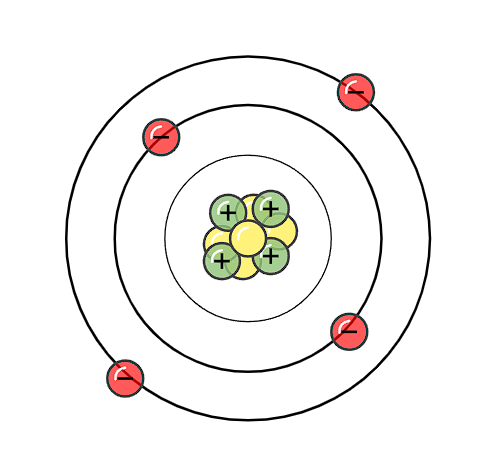
Protons: 4
Neutrons: 5
Electrons: 4
Locate the element Lead on your Periodic Table. Write it's:
Atomic Number, Symbol and Atomic Mass
Atomic Number: 82
Symbol: Pb
Atomic Mass: 207.2

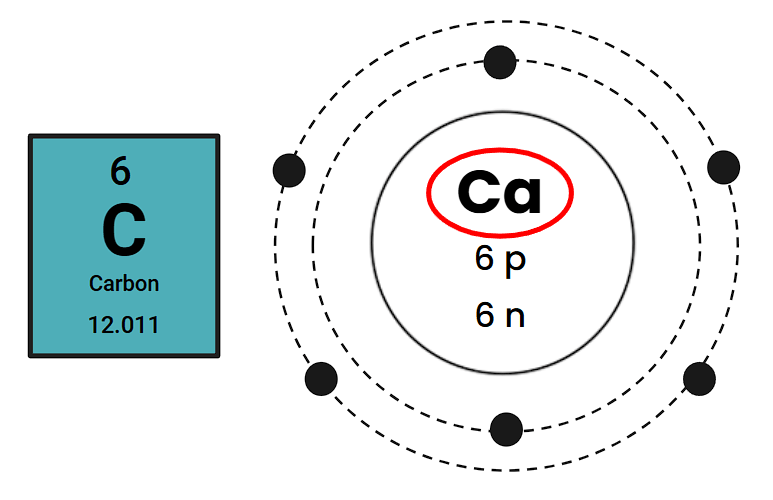
How many electron shells does this atom have?
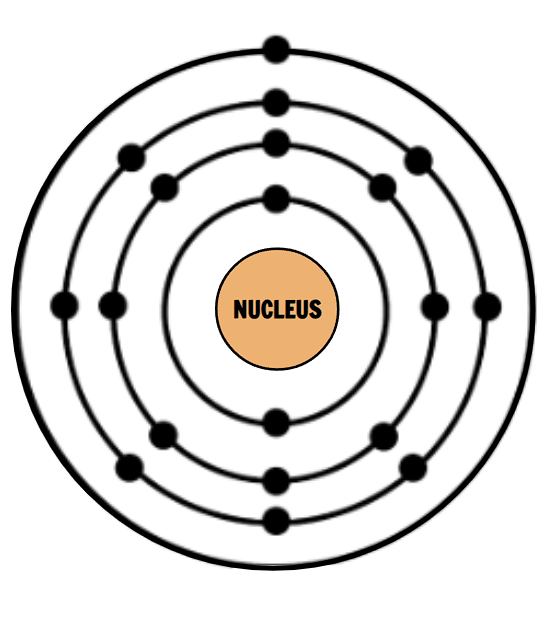
4 electron shells

Draw a Bohr Diagram for the element Beryllium:

Each group (columns) represents the number of:
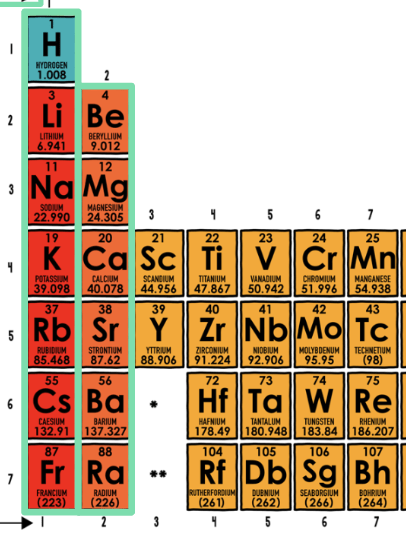
Valence Electrons