Which subatomic particles have an electrical charge of -1?
Electrons
What is this atom's atomic number?
2
What is this atom's atomic mass? Include units in your answer!

7 a.m.u.
What is lead's element symbol?
![]()
Pb
Without looking at your flashcards, which direction does a "group" on the periodic table go?
Vertical (up & down)
Which subatomic particles have a mass of 0 a.m.u.?
Electrons
An atom has 13 protons, 13 electrons, and 14 neutrons. What is its atomic number?
13
What is this atom's atomic mass? Include units in your answer!
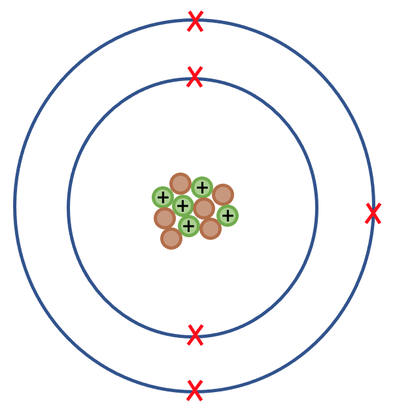
11 a.m.u.
What is manganese's atomic mass? Include units in your answer!
![]()
54.938 a.m.u.
Without looking at your flashcards, which vocabulary word has this definition?
"A chart that organizes the elements based on their properties"
Periodic Table
Which subatomic particles act like glue and help keep the nucleus stable?
Neutrons
Based on this atom's atomic number, which element is this? (Use your periodic table!)

Carbon
An atom has 8 protons, 8 neutrons, and 8 electrons. What is its atomic mass? Include units in your answer!
16 a.m.u.
How many protons does a chromium atom have?

24 protons
Without looking at your flashcards, which vocabulary word has this definition?
"A substance that is made of only one type of atom"
element
Which subatomic particles have a mass of 1 a.m.u.?
Neutrons and protons
An atom has 66 neutrons and an atomic mass of 115 a.m.u. What is this atom's atomic number?
#49 (Indium)
An atom has 35 protons, 45 neutrons, and 35 electrons. What is its atomic mass? Include units in your answer!
80 a.m.u.
Based on this element square, how many neutrons does a potassium atom have?
![]()
20 neutrons
Without looking at your flashcards, what is an "atomic number"?
the number of protons in that atom's nucleus, determines which element it is
Which subatomic particles determine an atom's atomic mass?
Neutrons and protons
An atom has 126 neutrons and an atomic mass of 209 a.m.u. Which element is this atom? (Give the element name!)
Bismuth (#83)
A Ruthenium atom has an atomic mass of 101 a.m.u. and an atomic number of 44. How many neutrons does a Ruthenium atom have?
57 neutrons
Based on this element square, how many neutrons does a Radium atom have?
![]()
138 neutrons
Without looking at your flashcards, what is an "atomic mass"?
the sum of protons and neutrons in an atom's nucleus