Dividing mass by volume tells you an object's what?
Density
Nitrogen
Explain whyIt's on the periodic table, so it must be an element, and elements are pure mixtures.
(Also, Nitrogen will possess the same properties no matter where you observe it)
A clump of calcium has a mass of 7.75 g and a volume of 5 cm3.
What is the density of calcium in g/cm3?
1.55 g/cm3

Baking Soda Volcano
Chemical Reaction
Gas is produced
Heat is produced
Which of the following can be observed without changing the chemical makeup of a substance?
a.) hardnessb.) flammability
c.) reactivity with oxygen
d.) toxicity
a.) hardness
If you can see all the different parts of a particular substance are not evenly mixed, you have one of these...
Heterogeneous Mixture
Dirt
Explain why
Heterogeneous Mixture
Well BP done did it again...A LARGE GASOLINE SPILL IS SEEPING INTO THE OCEAN!!!
The company executives have determined that have lost 25,000 g of gasoline. Gasoline has a density of 0.73 g/mL. How many mL of gasoline would be polluting the ocean? Round your answer to the nearest whole number.
34,247 mL

Simply biting a potato chip
Physical Change
All you did was change the shape of the chip.What are 4 signs that a chemical change has occurred?
- Produce heat or light
- Change in color
-Strong odor
-Precipitate
- Produce a gas
This solid forms from a chemical reaction where two liquids are mixed together
Precipitate
Disilicon Pentoxide
Compound
Two different element names can be picked out in this compound (nitrogen and oxygen), so these elements must be bonded together to create a compound.
A block of aluminum has a density of 5.4 g/cm3, a length of 5 cm, a width of 3 cm, and a height of 1 cm. What is the mass of the aluminum block in grams?
Your answer should be a whole number
81 g
 Baking a cake in the oven
Baking a cake in the oven
Chemical Change
Change of color
Strong odor produced
 A little nugget of sodium (d=0.971 g/cm3) is added to this display. With the following densities, predict where the nugget of sodium would float at in the cylinder.
A little nugget of sodium (d=0.971 g/cm3) is added to this display. With the following densities, predict where the nugget of sodium would float at in the cylinder.
Vegetable Oil= 0.92 g/mL
Corn Syrup= 1.4 g/mL
Milk= 1.033 g/mL
Dish Soap= 1.030 g/mLIt'd float towards the bottom of the vegetable oil and above the water
A type of substance where microscopic solids are dispersed in a fluid while maintaining their properties.
Colloid
Glue
Explain why
Homogeneous Mixture (colloid)
All of the different polymers/compounds in glue are spread out in water but retain their properties to produce a sticky texture
A phlebotomist draws 20 mL of blood from a blood donor. The density of blood is 0.00106 kg/mL. How many grams of blood did the blood donor donate? Round your answer to the tenths place.
21.2 g

Condensation
Explain whyPhysical Change
No heat or light produce, no change in color, or no precipitate. Water simply turns from a gas into a liquid.
Bonds are breaking and new types of bonds are forming to create new substances.
The process of separating two liquid by their boiling points
Distillation
Leaves on a tree
Explain why
Heterogeneous Mixtures
Different leaves may have different amounts of nutrients such as sugars and water.
What is the mass in kg of a 5-gallon jug of water?
18.9 kg
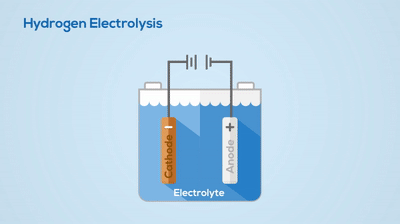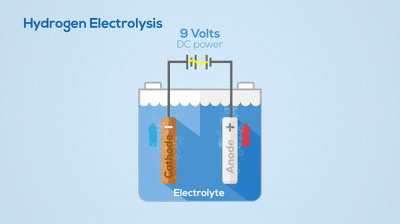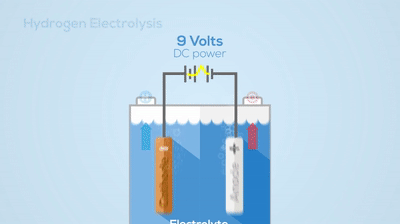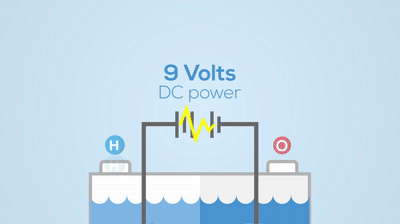
Electrolysis
Chemical Change
You can see the hydrogen and oxygen atoms leaving from the water, meaning a new substance has been created. This process is not easily reversed.Describe the basic differences between a pure substance and a mixture.
Pure substances are chemically bonded together and have the same properties no matter where they are found. Mixtures involved materials occupying the same space in a container, but they are not bonded together. Thus, the ratio of substances can change from mixture to mixture and create different properties.