Elimination
Elimination
Define the 4 pharmacokinetic parameters:
t1/2
Kel
Vd
CL
What is... elimination half-life
t1/2 - elimination half-life
Kel - First order elimination rate constant
Vd - apparent volume of distribution
CL - Clearance
What is the equation we use to find the elimination half-life?
What is...
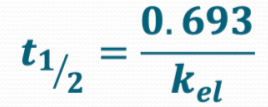
What does AUC mean in relation to drugs?
What is... it is the overall exposure of drugs to the blood.
What is bioavailability?
How do formulation and dosage form affect bioavailability?
What is...1. Protection from environment
2. Delayed/incomplete liberation from dosage form
3. Interference from excipients
What is...bioequivalence studies refer to relative bioavailability studies, which compare absorption from chemically equivalent dosage forms.
What is first-order elimination?
What is... Constant fraction eliminated per unit of time; more enzymes recruited as the plasma concentration rises.
What does Kel represent?
What is... fraction of drug eliminated per unit time; thus units are 'inverse time'.
What is AUC used to estimate?
What is...the extent of absorption.
What is... How fast? Rate of absorption
How much? Extent of absorption
How does the physiological state of a patient affect bioavailability?
What is... 1. Cardiac output affects hepatic circulation.
2. Disease state may affect GI motility
3. Population heterogeneity affects the expression of metabolic enzymes.
Why are bioequivalence studies performed?
What is...to compare the rate and/or extent of absorption of a new drug product or generic equivalent with that of a standard one.
What is non-linear elimination?
What is... Enzyme activity rises but not proportionally.
What is also known as "Saturation Kinetics"?
First-order kinetics
If you were given two graphs containing the profile of two different PO formulations (same dose) to the same patient, how would you know which formulation has less drug absorbed?
What is...The graph with the smaller AUC would mean less drug got absorbed.
What are the two types of bioavailability? Define them.
What is...absolute bioavailability: compare to IV dose, Ranges from 0 to 1.
Relative bioavailability: Compare to a recognized standard, less than/equal to/greater than 1.
State the 6 oral dosage forms from fastest to slowest bioavailability.
What is...
Solutions>Suspensions>Capsules>Tablets>Coated tablets>CR tablets
Define Bioequivalence.
What is...the absence of a significant difference in the rate and extent to which the active ingredient becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.
What is zero-order elimination?
What is... Constant quantity eliminated per unit time; enzyme activity is almost saturated.
Define t1/2
What influences the size of the AUC?
1. Size of dose administered.
2. How much of the administered dose gets into the bloodstream.
3. How quickly (i.e. at what rate) is the body able to remove the drug from the bloodstream.
What are the equations for absolute and relative bioavailability?
What are: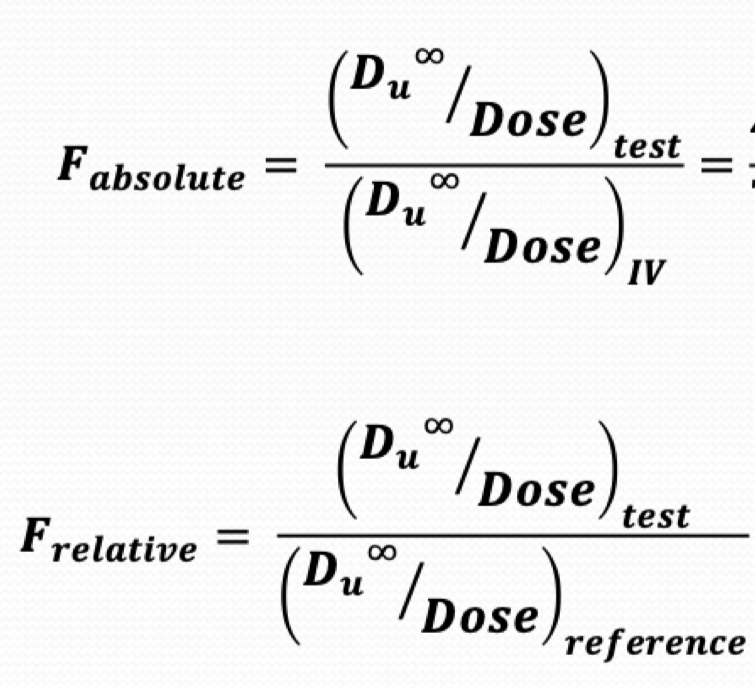
Bioavailability studies with pentobarbital from various dosage forms show that drug absorption rates decreased in what order. (State highest to lowest absorption rate)
What is...
Aqueous solution>Aqueous suspension of free acid>Capsule of the sodium salt>Tablet of the free acid
What components of a graph of drugs is used in bioequivalence testing?
What are... Cmax and tmax reflect the rate of absorption. Cmax and AUC reflect the extent of absorption.
How do zero-order and first-order elimination differ?
What is...
In zero-order the drug quantity eliminated per time unit is INDEPENDENT, while in first-order it is PROPORTIONAL.
In zero-order the amount of drug eliminated per unit of time is a constant QUANTITY, while in first-order it is a constant FRACTION.
Two other PK parameters that influence t1/2 are what?
What are...Volume of Distribution (Vd) & Clearance (CL)
*kel = CL/Vd
What is the trapezoidal Rule for AUC? What are the equations?
What is...Breaking the graph into individual trapezoids and then adding them together to get the total area.
What are... 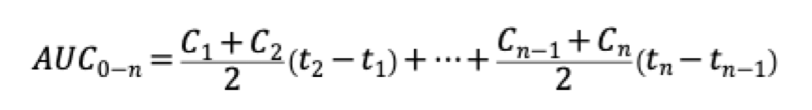
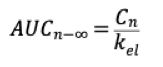
What is the bioavailable fraction? What are the components that make it up and the equation?
What is... fraction of the administered dose that reaches the systemic circulation.
Components: Fa - fraction absorbed, ffp - fraction escaping the first-pass effect.
Equation:
What are the values of graphs and tables that we look at and use to compare the bioavailability between drugs?
What are: ka and F on Table / tmax and Cmax on Graph
All these values give us AUC, and depending on F we can see which ones will have a higher AUC which - higher bioavailability.
How do we know that two products are bioequivalent? Percentage?
What is...when statistically significant differences are NOT observed in Cmax, tmax, and AUC.
A difference of <20% is the basis for bioequivalence.