What is a solid?
State of matter where the particles are very close together.
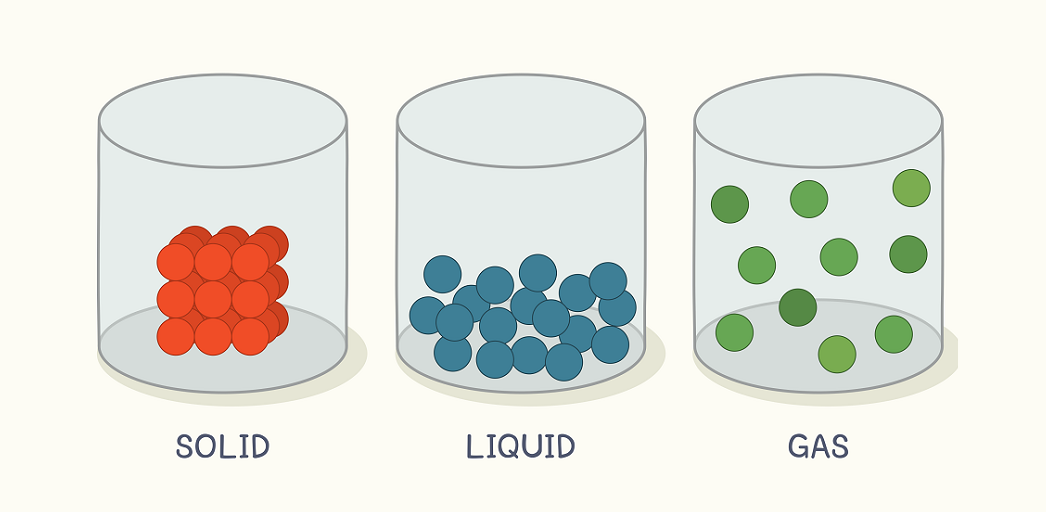
What is a Liquid?
State of matter where the particles are farther apart than solids but not as far apart as gasses.

What does Heterogeneous mean?
Diffferent
What does Homogeneous mean?
The same throughout.
What is matter?
Anything that takes up space and has mass.
What is a gas?
State of matter where particles are very far apart.

What is Plasma?
Fourth state of matter that is essentially pure energy that does not exist on earth.
State of matter where the matter has enough energy to overcome not just he attractive forces between its particles but also the attractive forces within its atoms.
What is the Law of Conservation of Mass?
Idea that the mass of all substances that are present before a chemical/physical change equals the mass of all the substances after the change.
What is a freezing point?
When a liquid turns into a solid.
What is a melting point?
When a solid turns into a liquid
What is Kinetic Theory?
Idea that all matter with an absolute temperature is made of small particles that are in constant motion.
Describe all four for a liquid:
What kind of density?
What kind of volume?
What kind of shape?
What strength of forces holding them together?
Medium density, definite/set volume, shape depends on container, medium forces holding them together.
What is a suspension?
Heterogeneous mixture made of a liquid and particles that do settle out.
What is boiling point?
When a liquid turns into a gas
What is condensation?
When a gas turns into a liquid.
Describe all four for a solid:
What kind of density?
What kind of volume?
What kind of shape?
What strength of forces holding them together?
High density, definite/set shape, definite/set volume, strong forces keeping them together.
Describe all four for gasses:
What kind of density?
What kind of volume?
What kind of shape?
What strength of forces holding them together?
Very low density, no definite/set shape or volume, and weak forces holding them together.
What is Colloid?
Heterogeneous mixture made of particles that never settle
What is Sublimation?
When a solid turns into a gas.
What is deposition?
When a gas turns into a solid
There are four key parts to Kinetic Theory. Describe two of them.
1. All matter is composed of tiny particles.
2. These particles are in constant motion, random motion.
3. These particles collide with each other and with the walls of any container in which they are held
4. The amount of energy that the particles lose from these collisions is negligible.
Come draw an accurate heating curve on the board.
teacher approval.
What is Distilation?
The process of separating substances in a mixture by evaporating a liquid and recondensing its vapor.
What is Heat of Fusion?
Amount of energy required to change a substance from a solid into a liquid at its melting point.
What is Heat of Vaporization?
Amount of energy required for a liquid at its boiling point to become a gas.