What is the most Electronegative element?
Fluorine.
This question is worth 200 points.
How would you make an atom/molecule negatively charged?
How would you make an atom/molecule positively charged?
Negatively: add electrons.
Positively: remove electrons.
200 point question.
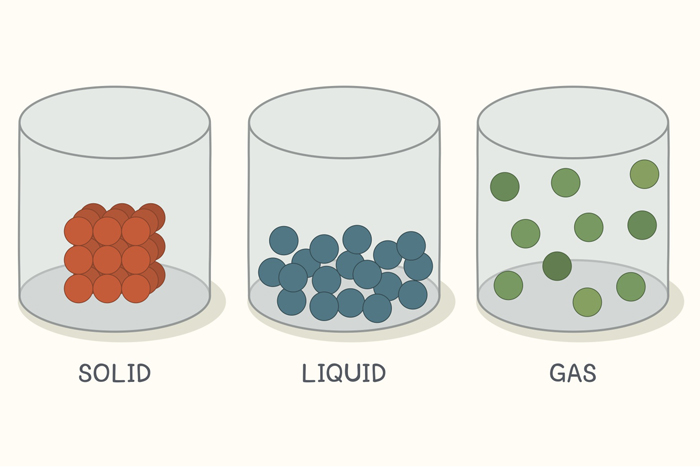
What are the four states of matter? Which one does not exist on Earth?
Solid, liquid, gas, and plasma
Plasma does not exist on Earth.
What is the only difference between a heating and cooling curve?
A heating curve the temperature increases, and a cooling curve the temperature decreases.
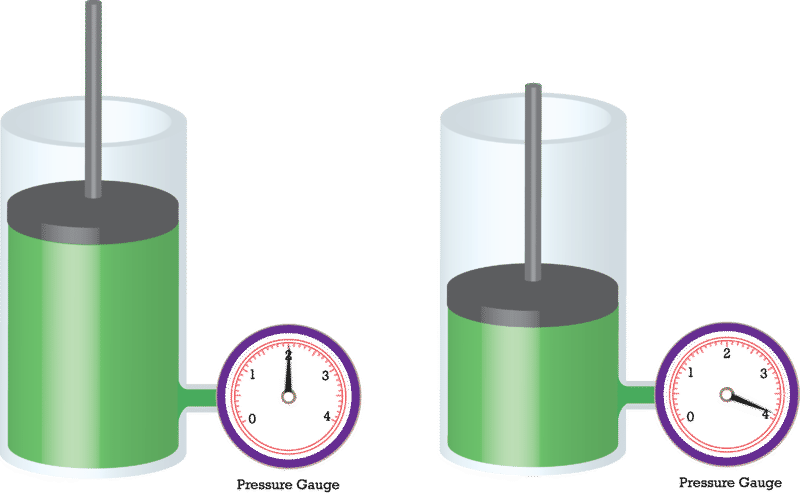
Which gas law is being represented here?
Constant temperature, change in pressure and volume.
Why do the Noble Gasses have the highest Ionization Energies?
They are very stable with 8 valence electrons and do not want to lose electrons.
What is the Octet Rule?
What happens to particle motion when you increase the heat (energy). Be specific.
You increase particle motion and change from one state of matter to another (ex. liquid to gas)
Explain boiling(evaporation) in terms of KMT.
The particles increase in energy and motion and go from a liquid to a gas.
Which gas law is represented here?
Constant Pressure, change in temperature and volume.
Is the Ionic Radius of an Anion bigger or smaller than a neutral/atom molecule? Explain.
Bigger. More electrons means the positively charged nucleus can't pull them in as strongly.
Is the Ionic Radius of a Cation bigger or smaller than neutral atom/molecule? Explain.
Smaller. There are less electrons for the positively charged nucleus to pull in so the nucleus can pull the whole atom in tighter.
Have someone from your team come up to the board and draw what the particles would look like in a solid, liquid, and gas.
Why does a gas create pressure on the walls of a container?
The gas particles inside the container are in constant motion and have higher energy so they are constantly hitting the walls of the container creating forces (pressure).
Which Gas Law is being represented here?
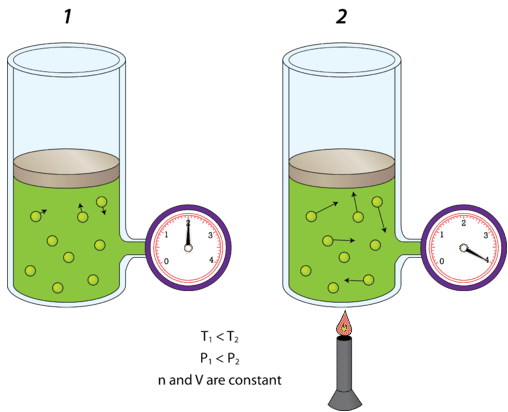
Constant Volume, change in temperature and pressure.
How does Atomic Radius relate to Ionization Energy?
Inverses of each other. A larger atomic radius means a lower Ionization Energy.
The larger an atom is, the easier it is to manipulate.
Based on the following reactions:
2Na + 2H2O -> 2NaOH + H2
Ca + 2H2O -> Ca(OH)2 + H2
Cl2 + H2O -> HClO + HCl
2Na + Cl2 -> 2NaCl
What would the reaction of Potassium and Iodine look like written in a chemical equation?
2K + I2 -> 2KI
What is the Kinetic Molecular Theory?
Idea that all matter with an absolute temperature is made of small particles that are in constant motion.
Have one member come up to the board and draw an accurate heating curve.
Teacher approval.
Explain freezing in terms of KMT.
Particles lose energy(heat) and slow down and cause a liquid to turn into a solid.
Based on the following reactions:
2Na + 2H2O -> 2NaOH + H2
Ca + 2H2O -> Ca(OH)2 + H2
Cl2 + H2O -> HClO + HCl
Ca + I2 -> CaI2
What would the reaction of Rubidium and Water look like written in a chemical equation.
2Rb + 2H2O -> 2RbOH + H2
A new substance has been found called Unobtainium (Un). The reaction pattern for Unobtainium with an Alkali Metal (AEM) is:
4(AEM) + Un2 -> 2(AEM)Un + 2(AEM)
Based on that hypothetical formula, What would the reaction of Lithium and Unobtainium look like in a written chemical equation?
4Li + Un2 -> 2LiUn + 2Li
What would a graph comparing the three phases of matter of the same substance at the same temperature look like? Come up to the board, draw and label it, and then explain the reasoning behind it.
Teacher approval.
How can KMT explain Diffusion?
Particles are in constant motion so when introduced to more volume the particles will spread out and evenly distribute.
Explain sublimation in terms of KMT.
Over a long period of time particles will randomly gain energy (heat) and transform from a solid into a gas.