Draw the Bohr model for neon atom.

What is wrong with this Bohr model?
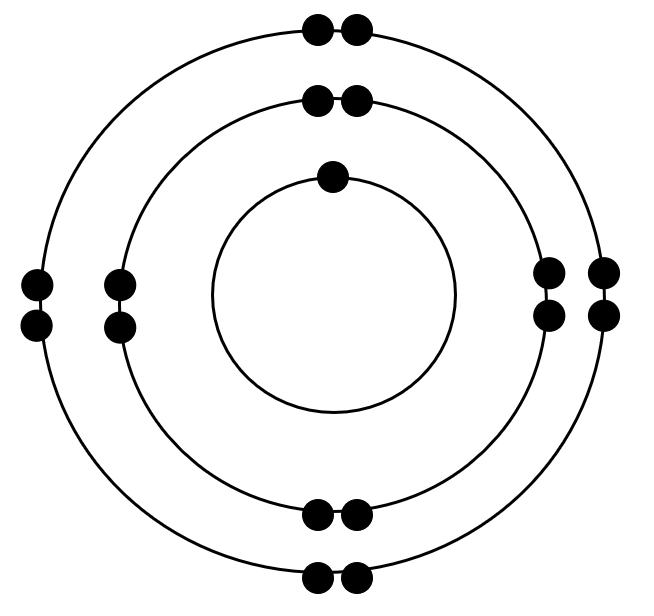
only 1 electron on first ring
What type of charge does neutrons have?
Neutral/ NO CHARGE
If an atom has 15 protons and 16 neutrons, what is its mass number?
31 g/mol
True/False: A cation is a positively charged ion.
True
Draw the Bohr model for chlorine atom.
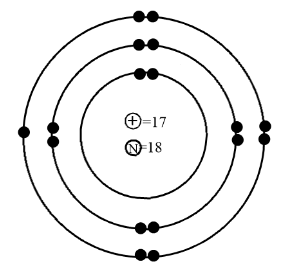
Who's Bohr Model is this?
chlorine atom
Where does the neutron belong?
In the Nucleus!
True or False: An element becomes an isotope when the number of protons changes.
FALSE!
How does an atom become a cation?
It Loses electrons!
Draw the Bohr model for calcium ion.
p = 20, n=20
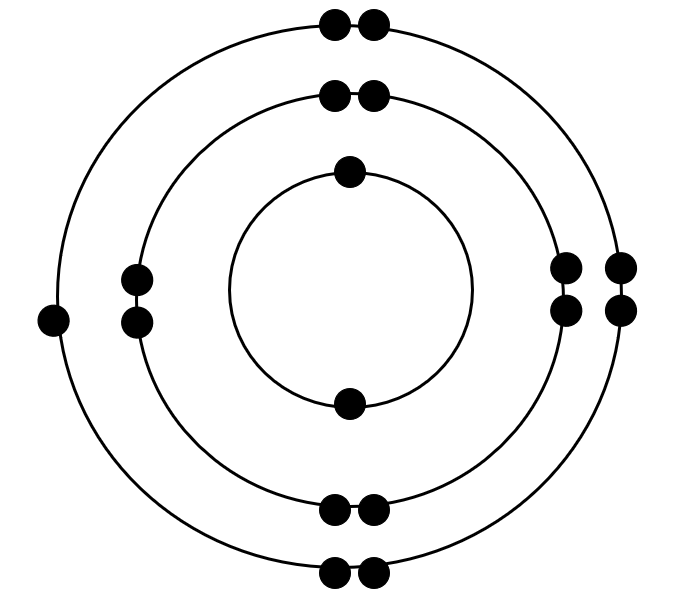
Who's Bohr Model is this?

Phosphorous atom
What do you call an atom that has LOST or GAINED an electron?
an Ion
What subatomic particle determines the identity of an element?
a proton!
Sodium (Na) forms an ion with a +1 charge. What happened to its electrons?
It lost one electron to become a cation.
Draw the Bohr model for potassium ion.
 n=20
n=20
Who's Bohr Model is this?
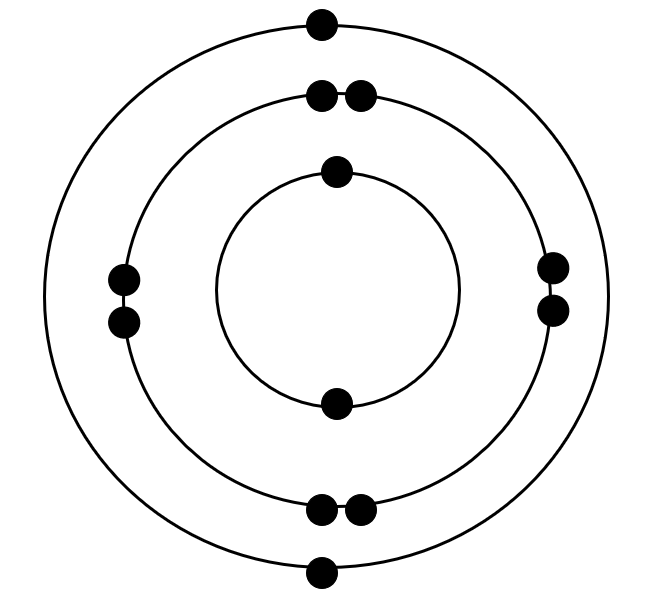
Magnesium atom
When a metal and a nonmetal bond, it forms an _____ compound.
Ionic
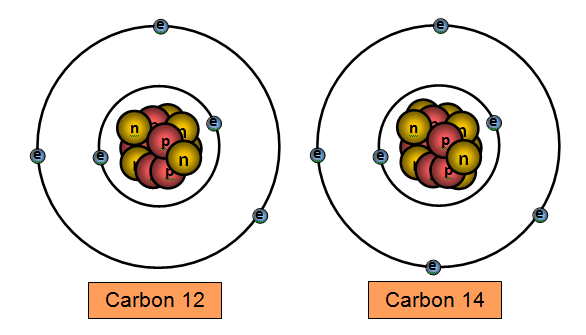
A B
If the main element is Carbon which one is the Isotope?
B
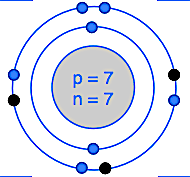
What is this element with its correct ion symbol?
N3-
Draw the Bohr model for aluminum ion.
p=13, n=14
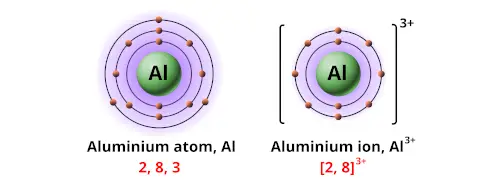
Who's Bohr model is this and how many protons and neutrons does it have?

Sodium atom, p = 11, n=12
What is a covalent bond?
When a nonmetal and nonmetal bond
A chlorine atom has a mass number of 37. How many neutrons does it have? (Cl has 17 protons.)
20 Neutrons
What ion is this? Include number of protons and neutrons in answer.

Calcium ion. 20 protons & 20 neutrons.