Draw the Bohr model for neon.
Who's Bohr Model is this?
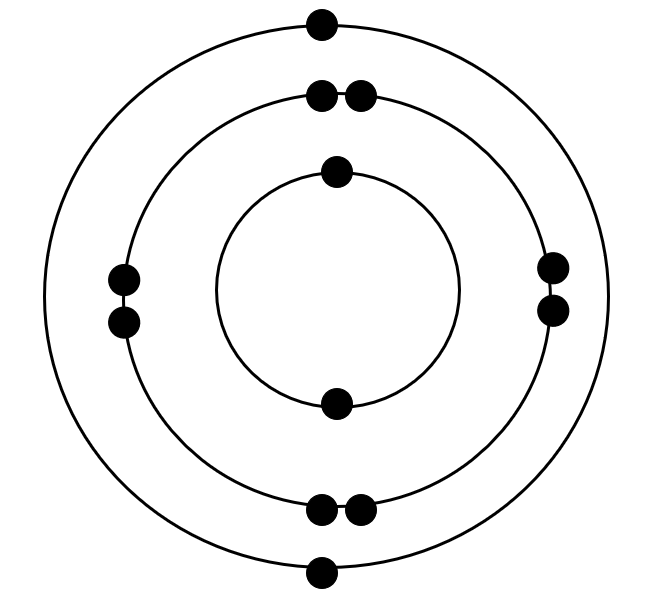
Magnesium
What are valence electrons?
electrons on outer energy level
What period and group is bromine in?
period: 2
group: 13
Draw the Lewis Dot Diagram for potassium.
Draw the Bohr model for chlorine.
Who's Bohr Model is this?
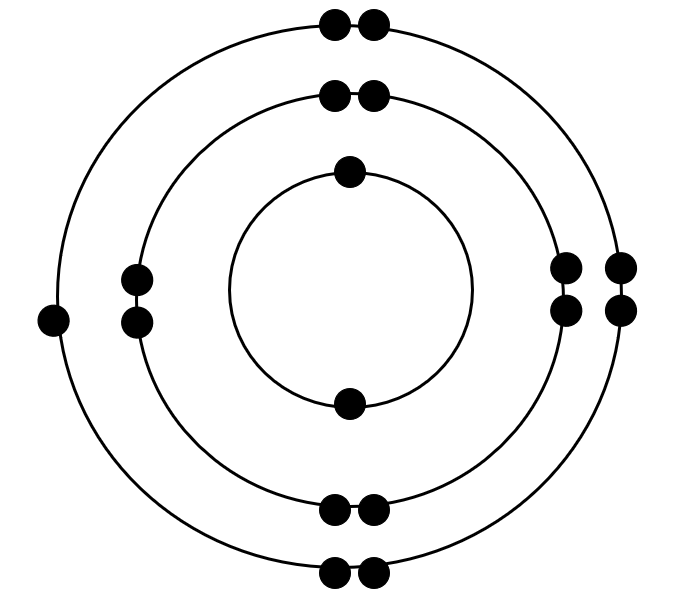
chlorine
How many valence electrons are in selenium?
6
What period and group is germanium in?
period: 4
group: 14
Draw the Lewis Dot Diagram for tin.
Draw the Bohr model for aluminum.
Who's Bohr Model is this?

zinc
How many valence electrons are in barium?
2
What period and group is helium in?
period: 1
group: 18
Draw the Lewis Dot Diagram for xenon.
Draw the Bohr model for potassium.
What is wrong with this Bohr model?
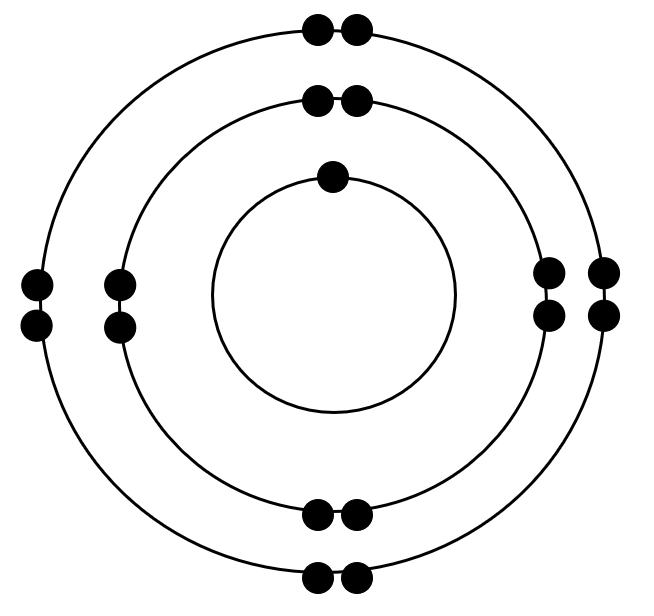
only 1 electron on first ring
How many valence electrons are in iodine?
7
What period and group is osmium in?
period: 6
group: 8
Draw the Lewis Dot Diagram for bismuth.
Draw the Bohr model for calcium.
What is wrong with this Bohr model?
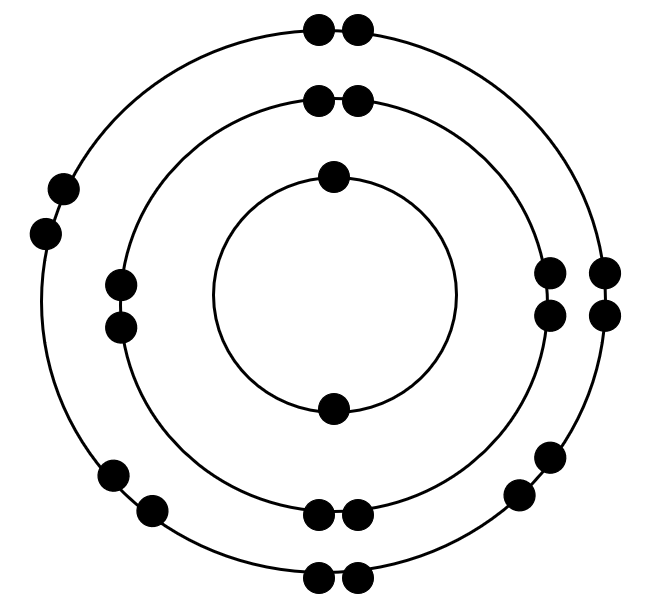
more than 8 electrons on outer ring
Why are valence electrons so important?
they participate in chemical reactions
What period and group is lead in?
period: 6
group: 14
Draw the Lewis Dot Diagram for helium.