The number of protons and electrons
What is the atomic number?
2n2
What rule do you use when determining the number of electrons in each shell/level?
electrons on outer energy level
What are valence electrons?
Columns of periodic table
What are groups?
Number of protons and electrons
What does atomic number tell you?
Rounded mass number - atomic number
How do you find the number of neutrons in an atom?
Number of protons and neutrons
What information goes in the nucleus when creating a Bohr model?
Outermost ring around an element's nucleus.
What is a valence shell?
Periods of the Periodic Table
What are the rows of the periodic table called?
Groups have similar properties (ie Noble gases, group 18)
What do elements in groups have in common?
Same elements that have different numbers of neutrons.
What is an isotope?
8 (for atomic numbers 1-20)
18 after Calcium
How many electrons are in the 3rd shell (or level) of an atom?
How many valence electrons are in Barium?
2
The number of valence electrons
What does the group number tell you (NOT counting transition elements)
Metals, non-metals, and metalloids. Based on location in the periodic table.
What are the 3 types of elements?
Average of the mass and abundance of each element's isotope.
Why are the mass numbers of the elements a decimal?
What is wrong with this Bohr model?
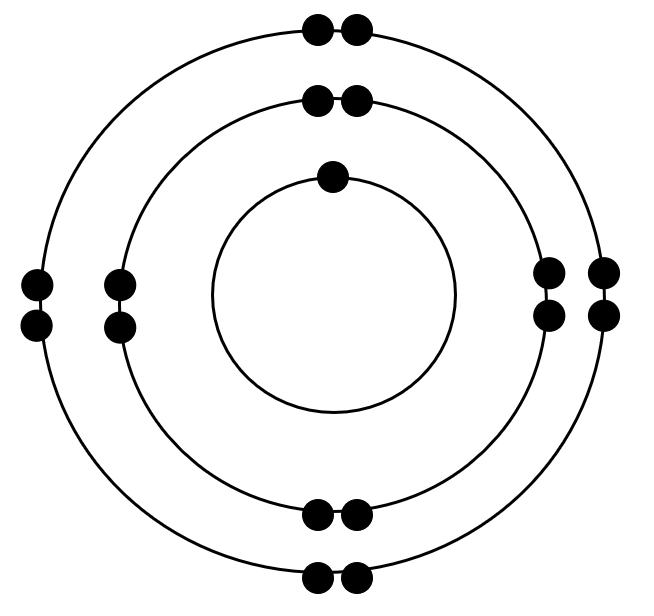
only 1 electron on first ring
How many valence electrons are in iodine?
7
Number of shells
What does the period of the periodic table tell you?
Number of shells in an element.
neutral (zero)
What is the charge of each element in the periodic table?
What is wrong with this Bohr model?
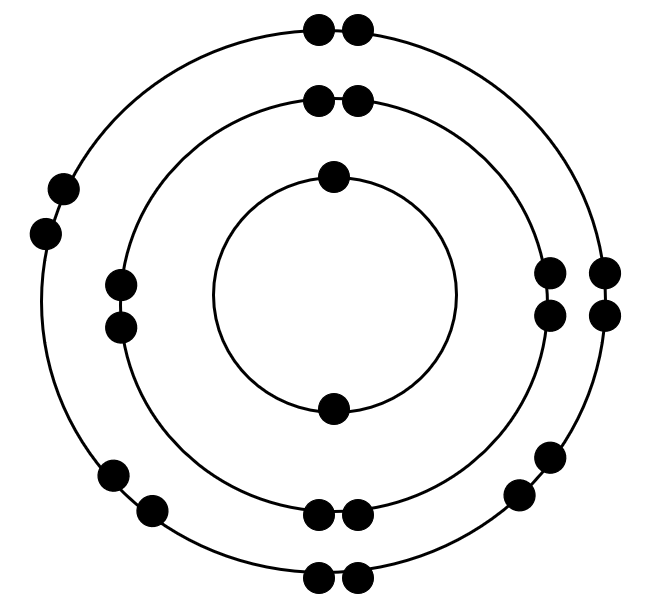
more than 8 electrons on outer ring
Why are valence electrons so important?
they participate in chemical reactions
Noble Gases.
What is Group 16 commonly called?
Number of valence electrons in an element.
What is the group number (minus transition elements)?