How many Total Valence Electrons does CH2O
A) 8 Valence Electrons B) 4 Valence Electrons C) 10 Valence Electrons
A) 8 Valence Electrons

How many bonds are used in total?
A)4 Bonds B)2 Bonds C)8 Bonds
A) 4 Bonds
N/A
N/A

What is the charge for NOF?
A) 6 B) 2 C) -2 D) 0
D) 0

What is the name of this compound and how many single bonds are there in this structure
A) Tetrachloromethane with 4 single bonds B) Dichlorocarbene with 6 C) Sodium Chloride with 8 bonds
A) Tetrachloromethane with 4 single bonds

How many double bonds are there?
A) 4 bonds B) 2 Bonds C) 5 Bonds
B) 2 Bonds
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How many Electrons are in this Lewis structure
A)12 Electrons B)8 Electrons C)6 Electrons
A) 12 Electrons in total
N/A
N/A
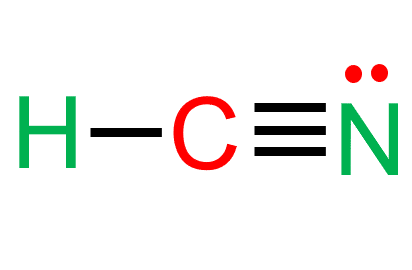 What is the molecular geometry of HCN
What is the molecular geometry of HCN
A) Tetrahedral Bent B) Triangular Planar bent C) Linear
C) Linear

What is the name of this lewis structure?
A) Tetrasulfure fluroide B) Tetrasulfure tetrafluroide C) Sulfur tetrafluroide
C) Sulfur tetrafluroide
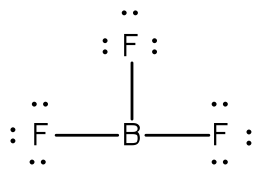
How many Valence electrons are there in total?
A) 24 Valence electrons B) 67 Valence electrons C) 20 Valence electrons
A) 24 Valence electrons
 How many Valence electrons does CO2 have?
How many Valence electrons does CO2 have?
A) 22 Valence electrons B) 18 Valence electrons C) 16 Valence electrons
C) 16 Valence electrons
N/A
N/A
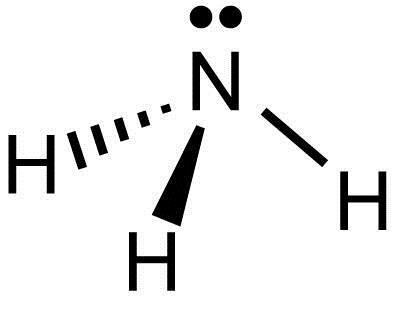
What is the Molecular shape of NH3?
A) Trigonal Pyramid B) Tetrahedral Bent C) Tetrahedral
A) Trigonal Pyramid
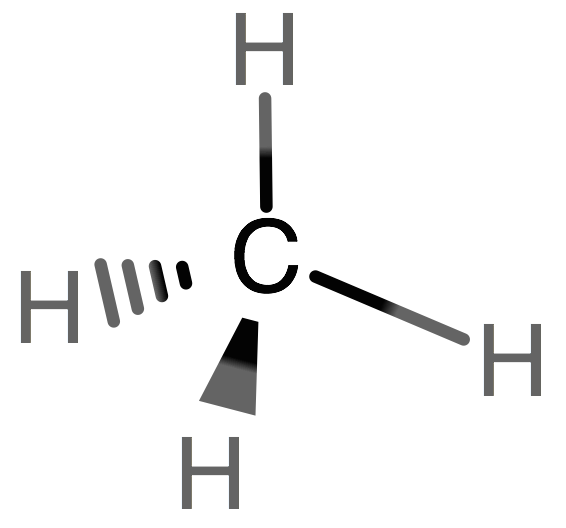
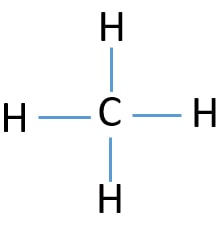
What is the Molecular geometry for CH4
A) Trigonal Pyramidal B) Tetrahedral bent C) Tetrahedral
C) Tetrahedral

How many double pairs are there in O2?
A) 1 pair B) 2 pairs C) 4 pairs
B) 2 pairs
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg) How many double bonds does H2CO
How many double bonds does H2CO
A) 4 double bonds B) 1 Double bond C) 2 Double Bonds
B) 1 Double bond
N/A
N/A

What is the molecular shape of NO2?
A) Linear B) Linear Bent C) Trigonal Planar
A) Linear, it is not bent because the lewis structure posted is an incorrect one, all you had to do was remove the lone pair from the N and just answer it
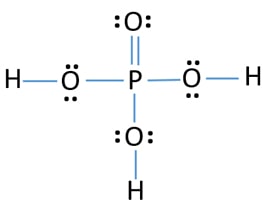
What is the Molar Mass of H3PO4?
A) 98.99 G/mol B) 97.99 G/mol C) 100 G/mol
B) 97.99 because, H3PO4 = 3(1.008 g) + 1(30.97 g) + 4(16.00 g) = 97.99 g

How many double pairs are there in CH2O
A) 2 pairs B) 4 Pairs C) 1 Pair
C) 1 Pair
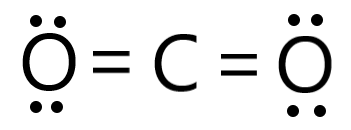
CO2 is considered bent because if the double bonds and because of the double of the lone pairs
A) True B) False
B) False, it is linear and lewis structures are considered bent if the middle had lone pairs
N/A
N/A

What is the molar mass of H4N2?
A) 14 g/mol B) 32 g/mol C) 12 g/mol
B) 32.0452 g/mol

How many lone pairs, how many double bond pairs, and how many Valence electrons are there?
A) 7 Lone pairs, 2 Double bond pairs, and 30 Valence electrons B) 2 Lone pairs, 1 Double bond pair and 36 Valence electrons C) 7 Lone pairs, 1 Double bond pair, 36 Valence electrons
C) 7 Lone pairs, 1 Double bond pair, 36 Valence electrons