Bond angle of linear
180
Bond that will form with N and H
polar covalent
H2O
Polar
H2O
Bond that involves the sharing of electron pairs between atoms
Covalent
Molecular geometry of AsF5
trigonal bipyramidal
Bond that will form between N and N
nonpolar covalent
CH4
Nonpolar
PCl3
Bond formed between two ions with opposite charges
Ionic
Bond angle that a tetrahedral shape forms
109.5
Bond that will form between Si and Cl
polar covalent
NH3
Polar
HCN

What is the mathematical product of the separation of the ends of a dipole and the magnitude of the charges?
Dipole moment
What does Valence shell electron pair repulsion (VSEPR) theorize?
The best arrangement of a given number of electron domains is the one that minimizes the repulsions among them.
Bond that will form between Si and F
Ionic
C2H4
Nonpolar
BF4-

This occurs when two or more equally valid electron dot structures can be written for a molecule
Resonance
Why does BF3 violate the octet rule?
Experimental evidence suggests that it is surrounded by 3 electron pairs and not 4.
Bond that will form between N and K
ionic
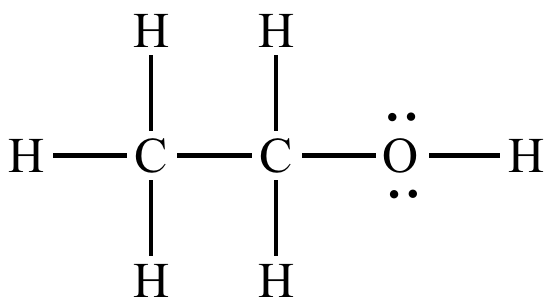
C2H6O
polar
C2H6O
What is the measure of the tendency of an atom to attract a bonding pair of electrons?
electronegativity
