What type of bond is described as the transfer of electrons?
Ionic
Roman numerals in a formula are used to indicate....
the oxidation state/charge on the positive ion
Which animals are stronger, penguins or polar bears?
polar bears
A molecule with an even distribution of charge is considered
nonpolar
This branch of the military flies planes
The type of bond between two nonmetals with different electronegativities
polar covalent
What is the name of the product in this reaction?
sodium chloride or sodium (I) chloride
Why does the second chemist die?
We changed our subscripts! (He drank H2O2!)
The type of bond where there is equal sharing of electrons
nonpolar covalent
According to science, opposites...
attract
The subatomic particle involved in bonding is the...
electron
What is the formula for titanium (IV) sulfide?
TiS2
Which animal do we eat?
FROGS! (Or fish if you're Sparky Parky)
Is this molecule polar or nonpolar?
Polar
Molecules with higher boiling points tend to have (stronger/weaker) intermolecular forces
stronger
007
James Bond
What is the name of (NH4)3PO4?
What happens to energy as a bond is broken?
energy is absorbed by the bond (BARF)
What does SNAP/OPEN stand for?
Symmetrical Polar, Asymmetrical Polar/ Odd Polar, Even Nonpolar
London dispersion forces are due to...
movement of electrons
Elements bond because they want to achieve stability. They do this by completing....
Their octet/ having 8 valence electrons
What is the name of Tc(PO4)2 ?
technetium (VI) phosphate
The bonding that is known for its "sea" of mobile electrons is...
metallic bonding
Which molecule has polar covalent bonds but is a nonpolar molecule?
2
The type of IMF shown here: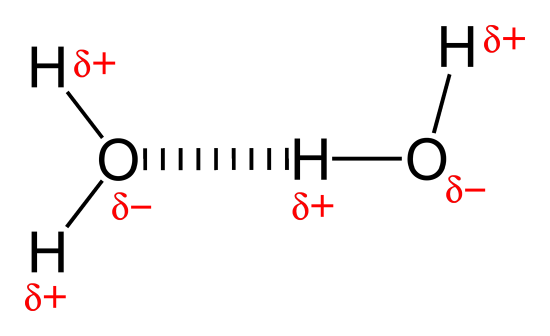
Hydrogen bonding