What is Disulfur dichloride?

S2Cl2
How do you say Ba3N2?
Barium Nitride
What is a chemical bond?
An attraction that binds atoms together
What is the octet rule?
The rule states that atoms gain, lose, or share electrons to have eight electrons in the outer shell
Positively charged ions are called...
Cations
What is Carbon Dioxide?
CO2

How do you say Fe2O3

Iron (III) Oxide
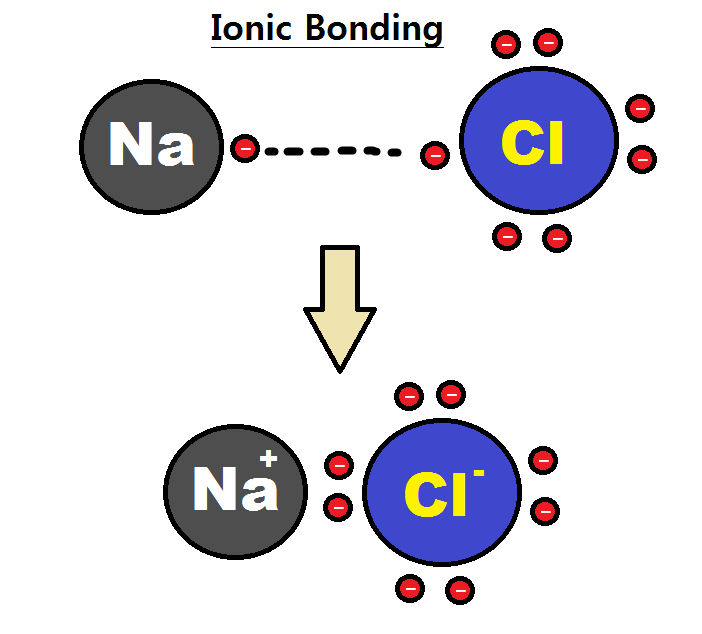
What is an ionic bond?
A bond between cations and anions
In a Lewis dot diagram, valence electrons are represented by

The dots placed around the symbol
Greek prefixes are used when naming...
Molecular compounds
What is Tellurium Decaflouride?

TeF10
How do you say Li2O?
Lithium Oxide
What is a covalent bond?
A chemical bond that involves the sharing of electron pairs between two non-metallic atoms
Does hydrogen follow the octet rule?
No
How are ions formed?
When atoms lose or gain electrons
What is Dinitrogen tetrahydride
N2H4
How do you say NH4Cl?

Ammonium Chloride
What is the difference between a polar and nonpolar covalent bond

Polar bonds have an unequal bond between electrons and nonpolar has an equal bond between electrons
By following the octet rule what happens to the atom?
It becomes stable
What is HgO
Mercury (II) Oxide
What is Dihydrogen monoxide?
H2O

How do you say CuSO4?
Copper sulfate
What is transferred in ionic bonding
Electrons
What group already has their valence shells filled
Noble gases

What is a valence electron
electron in the outermost energy level