What is the definition of electronegativity?
The strength of attraction for electrons by an atom
What two subatomic particles do you add together to determine the mass number?
Protons + neutrons = mass number
Name all six of the noble gasses
He, Ne, Ar, Kr, Xe, Rn
What is an isotope?
An element that has gained/lost neutrons
What is the periodic trend for atomic radius?
Atomic radius decreases from left to right across the periodic table.
Atomic radius decreases from bottom to top.
Atomic number on the periodic table tells you what?
The number of protons
Given the number of protons and neutrons, how would you determine which element you're looking at?
Count the protons! That's your atomic number, which determines your element.
If two nuclei have the same number of protons and different number of neutrons, what are they?
They are isotopes of the same element!
Salts are the colloquial word for what type of compound?
An ionic compound
(contains ionic bonds)
What are the periodic trends for electronegativity?
EN increases ACROSS (left to right) the periodic table and UP (bottom to top) the periodic table
Compared to an atom, would a cation (atom LOSES an electron) have a larger or smaller atomic radius?
A cation (positive ion) would have a smaller atomic radius than an atom because losing that electron causes the electron cloud to shrink.
A molecule must be nonpolar if it has a ________ charge distribution.
symmetrical!
How do you identify a salt?
i.e. What two kinds of atoms bond to form an ionic compound?
An ionic compound can be identified by a METAL bonded with a NONMETAL
Is H or O more electronegative?
Oxygen attracts electrons much more strongly.
Which group (column) of elements is the LEAST likely to undergo a chemical reaction?
The noble gasses are the least reactive!
In solid form, ionic compounds form what kind of structure?
Salts form a crystalline structure in an ionic form!
Think about how salt looks close up
Which list of elements is arranged in order of increasing electronegativity?
1. K, Ca, Sc
2. F, Cl, Br
3. Li, Na, K
4. Be, Mg, Ca
1. K, Ca, Sc
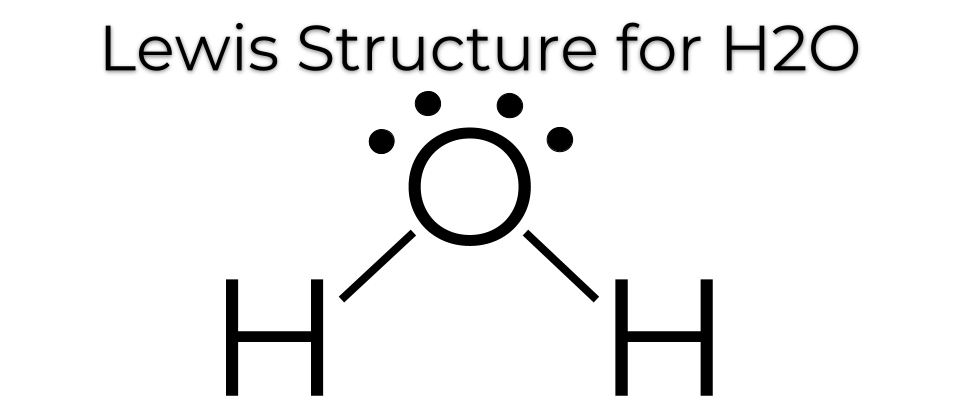
Draw the Lewis Dot Structure for H2O
Which of these two elements are the most chemically similar?
1. H and He
2. Be and Mg
3. K and Sr
4. P and S
2. Be and Mg
Because they have the same number of valence electrons. This is because they are in the same group!
[curveball: this isn't about isotopes]
If an atom forms a triple bond with another atom, how many electrons are they sharing?
6
The number of _________ in solution determines the electrical conductivity of an aqueous solution.
ions!