Covalent bonds form between___________ and ___________.
non-metal and a non-metal
Ionic bond form when electrons are ____________.
transferred
A link between atoms resulting from the mutual attraction of their nuclei and electrons.
What is a chemical bond?
NaCl
What is ionic?
What are lone pairs?
Shows the type & number of atoms in a SINGLE molecule of a molecule compound.
What is the molecular formula?
Metals losing an electron form a ________, while non-metals gaining an electron form a __________.
What is a cation & an anion?
These are the two major types of chemical bonds.
What are Covalent and Ionic?
MgS
What is ionic?
The shape of ...
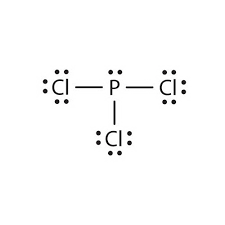
What is trigonal-pyramidal?
A covalent bond produced by sharing three pairs of electrons.
What is a triple bond?
Give the missing subscript
CaCl?
What is 2?
This calculation is used to determine bond type.
What is the difference in electronegativities?
SO4
Covalent
The shape of ....
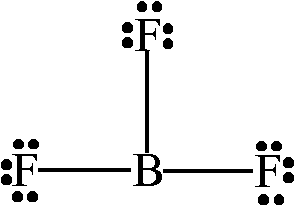
What is trigonal planar?
These use dots and dashes to represent the molecular compound like the following. (Looking for name of structure, NOT name of molecule or shape)
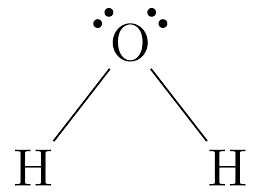
What is a Lewis (dot) structure?
What is the proper formula for Aluminum Chloride?
What is AlCl3?
These bonds have an uneven distribution/sharing of electrons.
What are Polar (covalent) bonds?
c6H12O6
What is covalent?
This is the shape CH4
What is tetrahedral?
Draw Lewis structure for the following molecule.
N2
(Bring your answer to the front)

This is the proper formula for Gold (II) Nitrate.
What is Au(NO3)2 ?
This is the type of bond between two oxygen atoms
What is double covalent bond?
Sodium Nitrate (NaNO3)
What is both
Which takes up more space- a lone pair or a bond?
What is a lone pair?