Triple bonds consists of how many electrons being shared?
What compound forms between Lithium and Phosphorus?
Li3P
Charge of Li is +1 and P is -3 so you cross them
Draw out Se2 Lewis Dot Structure
What is the VSEPR Shape for Se2
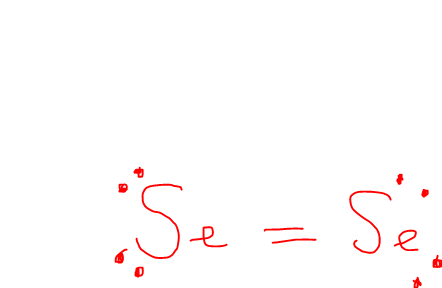 The VSEPR shape is Linear
The VSEPR shape is Linear
What is the chocolate you give to people to represent the X part of XOXO
Hershey's Kisses
What is the electronegativity of a polar covalent bond?
> 0.4 - 1.7
Ionic Bond
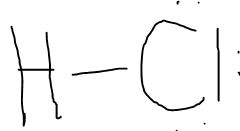
Draw out the Lewis Dot Structure of HCl
What does VSEPR stand for?
Valence Shell Electron Pair Repulsion
What type of roses do you get someone you're trying to FRIENDZONE!
Yellow Roses!
0, non-polar covalent
2 metals bonding together makes what bond?
Metallic Bond
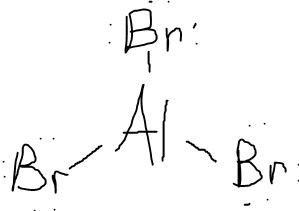
Draw out the Lewis Structure of AlBr3
Every Ionic Bond has one shape, what shape is that?
Crystalline/Crystal
Singles Awareness Day/National Singles Day!
Give the electronegativity of Ni-O, and what type of bond is it? (Be specific)
1.7, Polar Covalent
These types of bonds have opposite ends?
What compound forms between Magnesium and Oxygen
MgO
Charge of Mg is +2 and O is -2 so you cross them and then simplify!
Draw out and determine the Shape of SiBr4
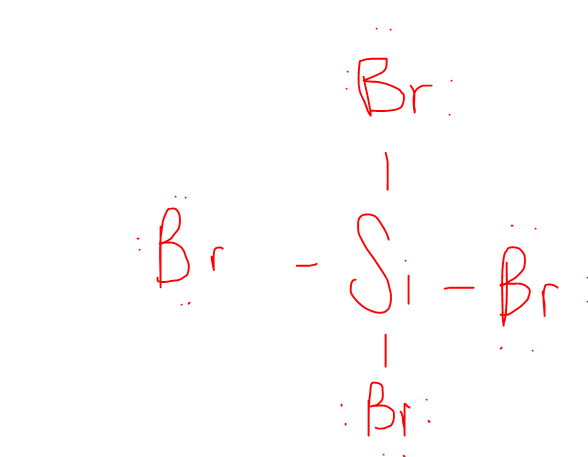 The VSEPR Shape is Tetrahedral / Non-Polar
The VSEPR Shape is Tetrahedral / Non-Polar
Who is the Roman God of desire, attraction and affection?
Cupid/Eros
What is the electronegativity of Fe-Au, and what type of bond is it?
.6, Metallic!!!!
What is the list of Diatomics?
BrINClHOF
Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, Flourine
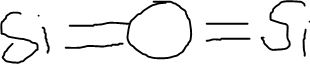
Draw out the Lewis Dot Structure of Si2O
What is the VSEPR Shape of SCl2?
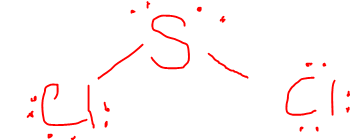 The VSEPR Shape is Angular/Bent / Polar
The VSEPR Shape is Angular/Bent / Polar
Everyone knows the beginning of the modern version of the poem "roses are red, violets are blue" but what is the next verse?
Sugar is sweet, and so are you