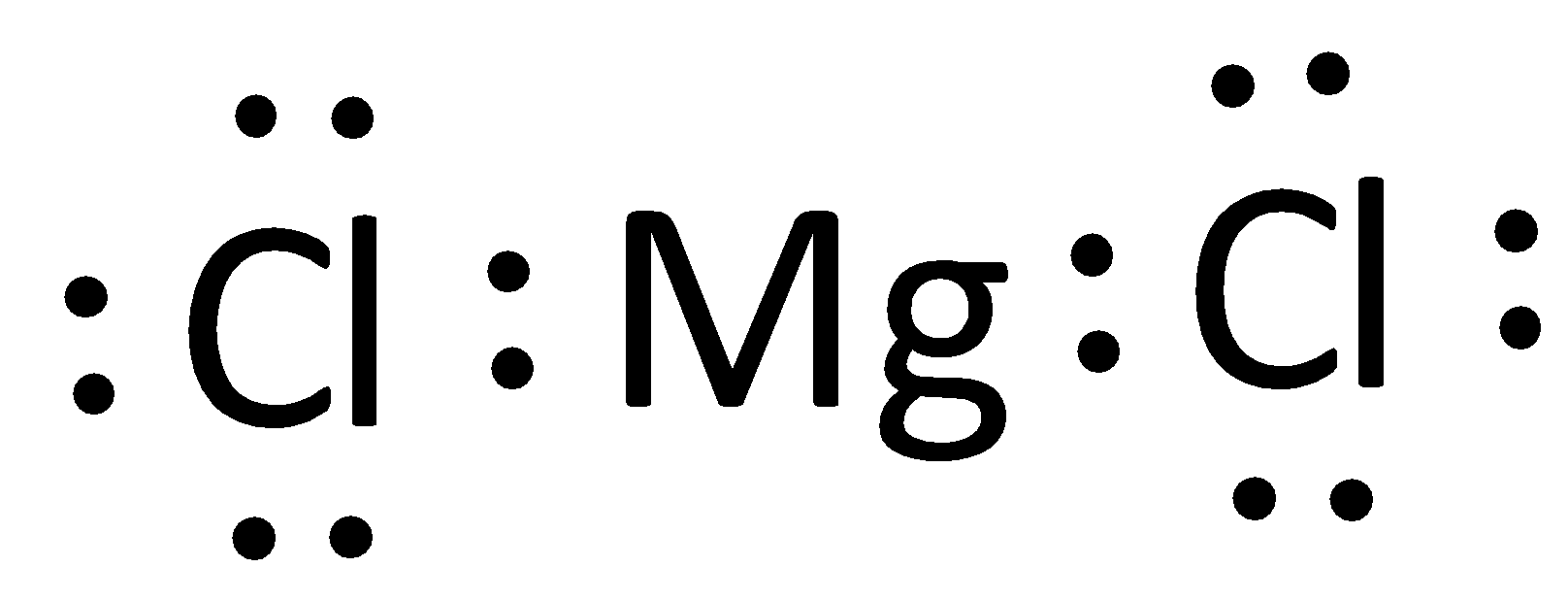The chemical formula for ammonia (Nitrogen (N) and Hydrogen (H)
What is NH3?
Metallic bonds are formed between elements from this category on the periodic table.
What are metals?
Covalent bonds are formed between this category of elements.
What are nonmetals?
This is the name for a negative ion.
What is an anion?
The Bohr model for Hydrogen

The chemical formula for sodium chloride (Na and Cl)
What is NaCl?
Metals conduct electricity in this state of matter.
What is a solid?
Polar covalent bonds have this type of electron sharing.
What is uneven/unequal electron sharing?
Ionic bonds are formed between these categories of elements.
What is a metal and a nonmetal?
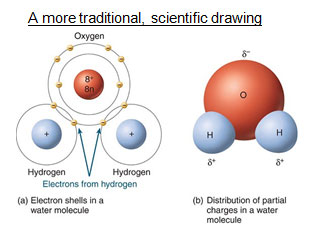
A water molecule
The chemical formula for lithium oxide (Li and O).
When mixed with water, metals do not undergo this process- unlike ionic bonds and some covalent bonds.
What is dissolve?
Nonpolar covalent bonds have this type of electron sharing.
What is equal/even electron sharing?
The name for a positive ion.
What is a cation?
The Lewis dot diagram for sodium chloride (NaCl)

The chemical formula for water
What is H2O?
Metals conduct electricity as a solid thanks to having this unique characteristic.
What are free-flowing electrons?
In water, this element has a higher electronegativity.
What is oxygen?
Ionic compounds do this when dissolved in water as a solution.
What is conduct electricity?
The Lewis dot diagram for Magnesium Chloride (MgCl)