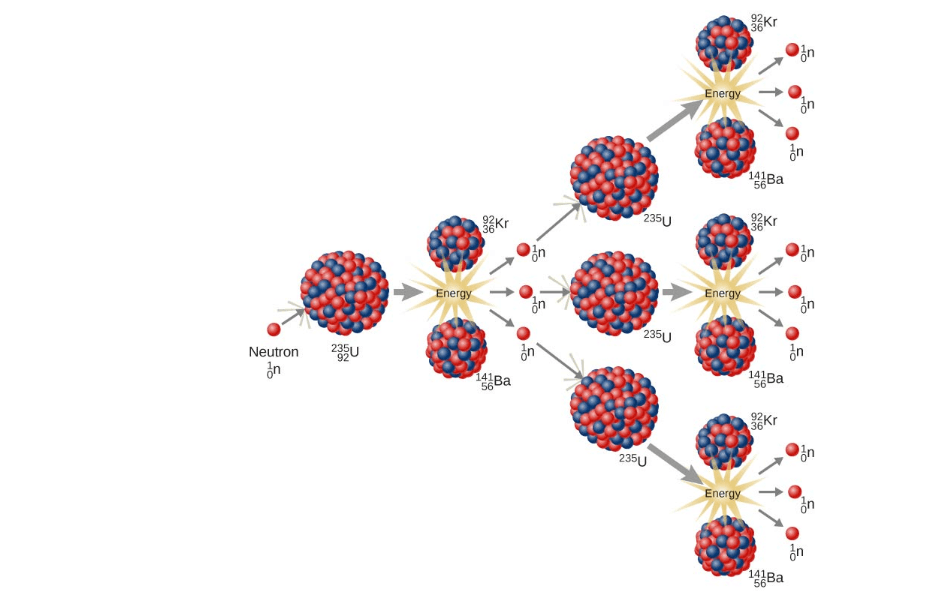
Fission
In an endothermic reaction, hot or cold temperatures are realeased
Cold
Random motion of particles
thermal energy
a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles
In the following reaction, energy is
N2 + 3H2 ---> 2NH3 H = -92.2 kJ
Released
heat
What is the atomic mass of the new element?
23892U ---> 42He + 23490Th + 00y
234
In the reaction below, how many joules of heat are needed to react with 2.00 moles of oxygen?
2CO + O2 ---> 2CO2 + 134.5 joules
269 J
A log burning is an example of these TWO kinds of energy
Chemical and thermal
What is the mass of the missing particle?
94Be + 42He ---> _____ + 10n
12
In a calorimeter holding 500 g of water the temperature changes from 10 C to 48 C. How many kilojoules of heat were added to the water? Specific heat of water is 4.18 J/gC
79.42 kJ
Law of conservation of energy definition
Energy cannot be created or destroyed; Energy before and after a reaction must be equal
Fill in the blank
23090Th ------> 0-1e + _______
Given the equation,
C2H5OH + 3O2 ---> 2CO2 + 3H2O + 312 kJ
what amount of energy is released when 2 moles of oxygen completely reacts?
208
A rock balanced at the top of a hill is _____ energy
potential