You can sweep broken glass into the trash with your hand as long as you have gloves on.
True or False
False, never touch broken glass with your hand, gloved or ungloved
What is the formula and units for density?
A. Volume ➗ mass (milliliters/grams)
B. Mass x volume (grams/density)
C. Mass ➗ volume (milliliters/grams)
D. Mass ➗ volume (grams/milliliters)
Mass ➗ volume (grams/milliliters)
Double or nothing: What's the abbreviation for grams/milliliters?
Label A and B as accurate or precise

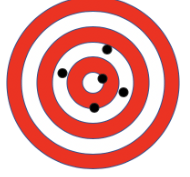
 Precise, not accurate
Precise, not accurate
 Accurate, not precise
Accurate, not precise
Give a definition for intensive and extensive properties.
Intensive properties do not depend on how much you have. Extensive properties do.
Double or nothing: How do you remember the difference between the two?
Solubility (how well a substance dissolves). Explain
Physical because dissolved substances can be reversed without a chemical change.
You only need goggles when dealing with dangerous chemicals.
True or false
False, you need to protect your eyes whenever you are performing any lab.
Calculate density for an object with a mass of 14 g and a volume of 7 cm3
what is 2 g/cm3
Give a definition of precision and accuracy
Precision is how close a series of measurements are to each other.
Accuracy is how close measurements are to the true value.
Intensive or Extensive property? Explain.
Brightness of laptop screen
Intensive
Odor, explain why.
Physical, because there is no chemical change in the chemical that causes smell.
It's okay to mix chemicals together in the lab without the teacher's permission, as long as they are safe.
True or False?
False
Even if you think that mixing chemicals together is safe, don't...
Calculate density: Volume is 100 ml and mass is 25g. Must include correct units
what is 0.25 g/ml
A typical bag of skittles contains 59 pieces of candy. Judy counts her bag and finds 57, Josh finds 58, and Jill finds 59. The company is...
A. accurate B. precise C. accurate & precise
D. neither accurate nor precise
C. accurate & precise
400 more points: If the mass of the skittles bag is 2.17 oz. Make a series of 3 numbers that are accurate and precise. Each number must include 2 decimals.
Intensive or Extensive property? Solubility (dissolving one thing in another) and explain why.
Intensive, because a substances ability to dissolve does not depend on how much there is.
Hair texture, explain why
When an object changes color without undergoing a chemical change, it is still considered a physical property.
You always add acid to water because if you do it the other way around, a large amount of heat is generated, and both the acid and water could splash on nearby bystanders. Is this an example of a chemical or physical change? Explain.

Chemical. When water reacts with an acid, heat is generated.
Which two instruments do you need to measure the density of a liquid?
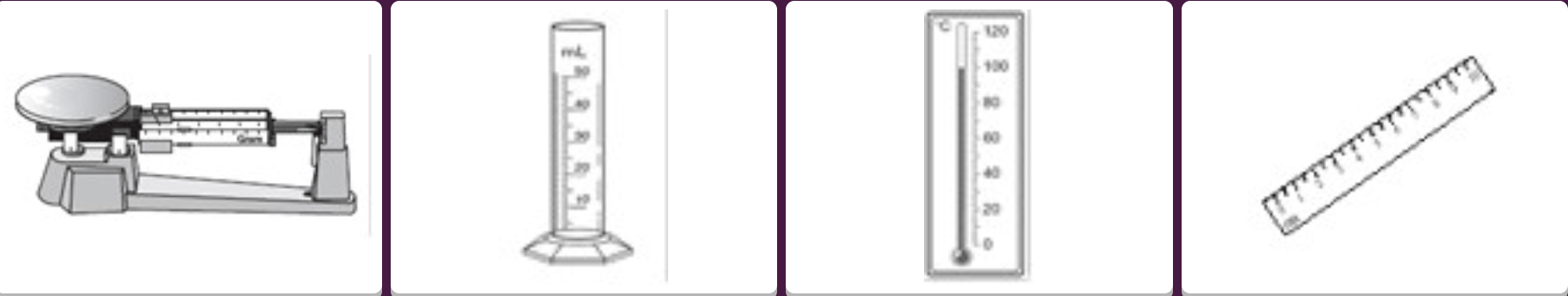
A and B
Double or nothing: Name these two instruments!!
True Value: 25.01;
Measurements: 24.94, 25.22, 25.13, 24.08;
These measurements are…
A. Accurate, but not precise
B. Precise, but not accurate
C. Both precise and accurate
D. Neither precise nor accurate
C. Both precise and accurate
Temperature of cell phone after using it for an hour. Explain why.
Intensive, because temperature doesn't depend on how much matter you have.
Sound, explain why
Sound is a physical property because the waves that travel through air to create sound does not create a new substance.
You're performing a lab experiment with your lab partner, and a chemical splashes on your skin. You look up, and the teacher is nowhere to be found. What's the first thing that should you do?
A. Wait for the teacher to get back
B. Go to sink and rinse off for 5-10 minutes
C. Run outside and look for an adult
D. Go to the sink and rinse off for 15 - 20 minutes
D. Go to the sink and rinse off for 15 - 20 minutes
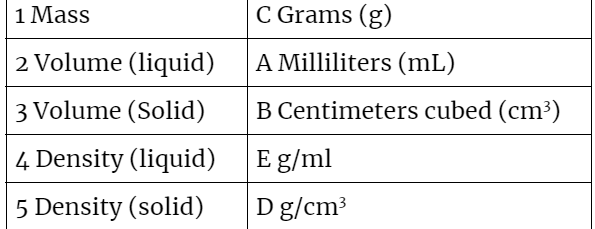
Match the measurements on the right with the correct units on the left.
Student C
+250 pts.: which student has the most accurate results?
Intensive or extensive and why?:
Energy present in a in an explosion.
Use the following equation to answer:
Energy = mass x speed2
Extensive, because it depends on how much mass.
Water's reflective properties. Explain why
A substance's reflection can be observed without changing it to another substance, including water.
