Histology, Pathology and Tissue
Actual project deliverables and task responsibles are defined in this document.
A) What is QMS07?
B) What is the Design and Development Plan (DDP) (TX20000)?
C) What is the System Requirements Specification (SRS) (TX20210)?
A) What is QMS07 (Product Development and Design Control)?
This QMS document outlines the model describing the process of starting and performing product development, product design changes and product transfer to manufacturing in compliance with governing regulations.
A) What is QMS03?
B) What is QMS05?
C) What is QMS07?
C) What is QMS07?
This pre-analytical variable is the primary reason for breast/fatty tissues falling off the slide during any type of staining.
A) What is incorrect processing protocol used?
B) What is cold temperature?
A) What is incorrect processing protocol used? (fatty tissues need a longer processing schedule for complete infiltration of paraffin)
This is the full name of OCMO.
A) What is Office of the Chief Medical Officer?
B) What is Organization of the Chief Medical Office?
C) What is Organization of the Chief Medical Officer?
C) What is Organization of the Chief Medical Officer?
Name this project management information system.
What is Clarizen?
The acronym BCSL stands for this.
What is the "Biopharma CDx Services Lab"?
True or false?
Laboratory Information Management System (LIMS) facilitates the management of samples, test results, and associated data to improve lab productivity and efficiency?
True
The total range of measurable values or concentrations of an analyte that can be detected by a particular assay.
A) What are continuous measurements?
B) What is the total concentration of values?
C) What is the dynamic range?
C) What is the dynamic range?
This is the most common slide type used for H&E staining in US clinical labs.
A) What are charged slides?
B) What are non-charged slides?
B) What are non-charged slides?
This country requires a local ring trial and reader reproducibility study for CDx registration.
A) What is the EU?
B) What is China?
C) What is Japan?
D) What is Russia?
B) What is China?
This type of expense is invoiced to a pharma partner with no profit margin for Agilent.
A) What are FTE hours?
B) What is a pass-through expense?
C) What are right of use fees?
What is a pass-through expense?
This is the city where the ASCO annual meeting takes place.
What is Chicago?
This is who you should report in to when you are asked to participate in an audit of a CDx project/product.
A) Who is the project manager?
B) Who is the front room manager?
C) Who is the back room/office lead?
C) Who is the back room/office lead?
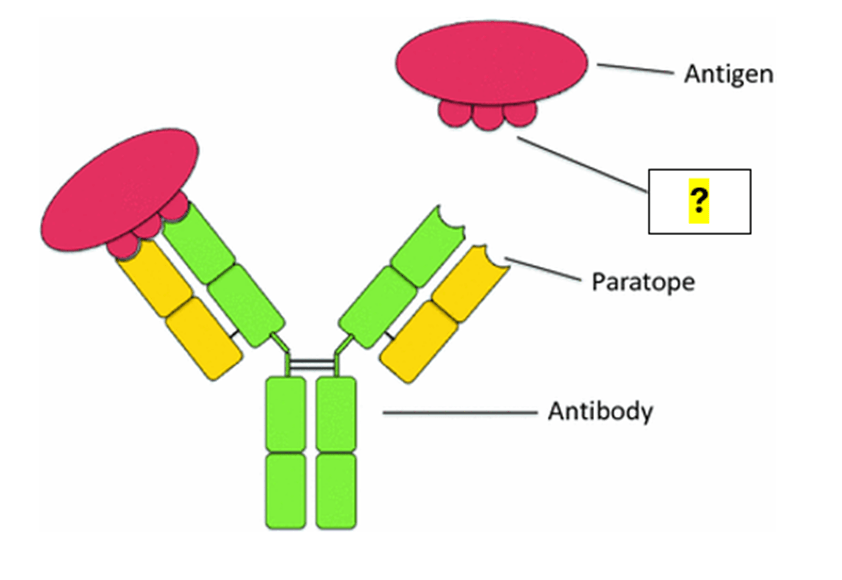
A) What is a T-cell?
B) What is an Epitope?
C) What is an Inhibitor?
D) What are Phalanges?
B) What is an Epitope?
This type of cell can be found in well differentiated squamous carcinomas and resembles something for breakfast.
A) What is an omelet cell?
B) What is a cheerios cell?
C) What is a yogurt cell?
D) What is a fried egg cell?
D) What is a fried egg cell?
The below screenshot is from this type of FDA meeting which occurred on September 26, 2024.

A) What is a Pre-Sub meeting?
B) What is an ODAC meeting?
C) What is a Type C meeting?
B) What is an ODAC meeting?
This type of code is a reportable part of the project plan where resources are assigned.
What is a T-code (task code)?
This indication is approved for use with product code GE006 (PD-L1 IHC 22C3 pharmDx Dako Omnis).
What is NSCLC?
These 3 different types of CDx studies each require documentation of protocol deviations on their own specific templates.
A) What are feasibility, verification/validation, and development studies?
B) What are stability, verification/validation, and clinical studies?
C) What are marketing, clinical studies, and ring trial studies?
B) What are stability, verification/validation, and clinical studies?
This is the recommended number of specimens used in a standard cut-section stability study.
A) What is 4?
B) What is 6?
C) What is 8?
D) What is 10?
B) What is 6?
These cells can be found in all tissues. They are usually single and can have granules in the cytoplasm. They are tissue vacuum cleaners and are part of the immune response.
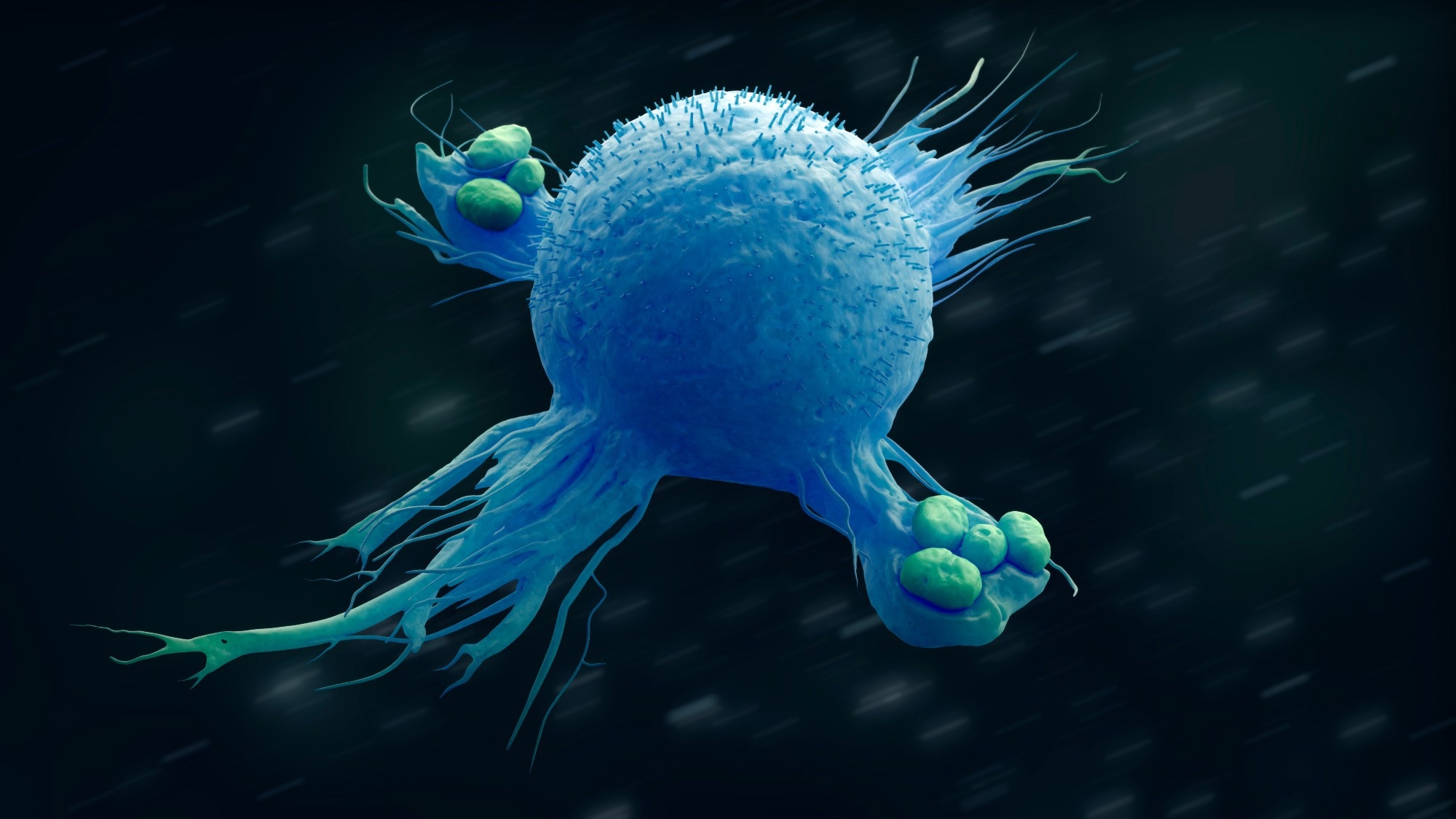
A) What are Leukocytes?
B) What are Neutrophils?
C) What are B-Cells?
D) What are Macrophages?
D) What are Macrophages?
This type of submission may be required to submit in each EU country for retrospective testing of European clinical trial samples using Agilent's IUO/PEO kits for the purpose of clinical bridging.
A) What are CDx notifications?
B) What are Performance Study Applications?
C) What are Conformity Assessments?
A) What are CDx notifications?
This person is responsible for estimating the amount of work left to complete a task.
Who is a Remaining Effort Owner?
This assay was Agilent’s most recently approved CDx in the US
What is MAGE-A4 IHC 1F9 pharmDx? (MAGE-A4 counts)
Guidelines for how to route non-QMS documents in Agile are found in this QMS document.
A) What is TP20050?
B) What is TP20220?
C) What is TP20216?
C) What is TP20216?
This equation mathematically correlates the increased temperature of an accelerated reagent stability test with real time equivalent in days at the standard storage temperature.
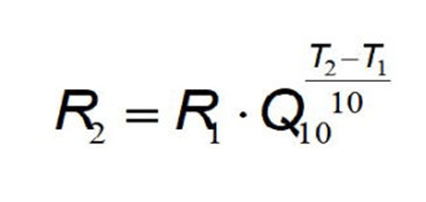
A) What is the Q-Rule Equation?
B) What is the Binomial probability distribution?
C) What is an Isochronous design?
A) What is the Q-Rule Equation?
Choose three reasons for poor tissue section quality that is related to the tissue block (not instrument or Histologist) that makes them difficult to section.
What are....
-Poor fixation
-Poor shipping condition
-Calcifications
-Long storage time
-Poor tissue processing
poor fixation, poor tissue processing, and calcifications
Performed by CAS, this is the trending analyses of device-related data in a clinical trials to identify unexpected trends and discrepancies.
A. What is Clinical Monitoring?
B. What is EDC?
C. What is Instream Data Monitoring?
C. What is Instream Data Monitoring?
This type of chart illustrates a project's work break down structure.
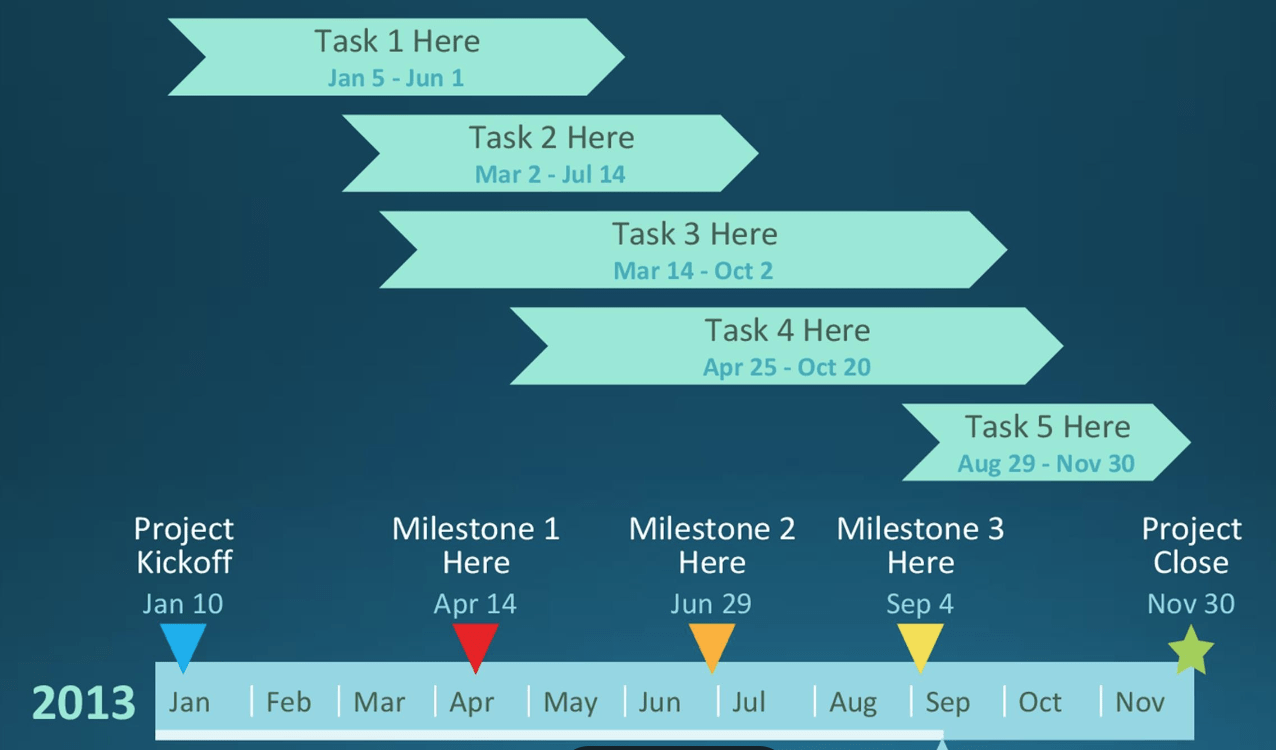
What is a Gantt chart?
This is the product code for the PD-L1 22C3 Antibody Concentrate available in China
What is M3666?