What are the charges and placement of subatomic particles (N0, e-, p+)?
Neutrons and protons in the nucleus, electrons in a cloud around nucleus; protons have +1 charge, electrons -1, neutrons neutral
Convert 20.5 cm3 to m3.
2.05 x 10-5 m3
What is the equation that relates wavelength and frequency of electromagnetic radiation?
𝜈 = c/λ
What properties distinguish solids from liquids? Liquids from gases? Solids from gases?
Solids: unchanging dimensions, unchanging volume
Liquids: changing dimensions, unchanging volume
Gases: changing dimensions, changing volume
What is the difference between a molecular and empirical formula? Give an example of each.
Molecular formula = accounts for all atoms in molecule; Empirical = atoms in smallest whole # ratio
What are the diatomic elements?
H2, N2, F2, O2, I2, Cl2, Br2
Given the ion with a 3+ charge, 28 electrons, and a mass number of 71, write out the isotope formula
7131Ga3+
What is the mass of 50.0 mL of octane, with a density = 0.702 g/cm3?
35.2 g
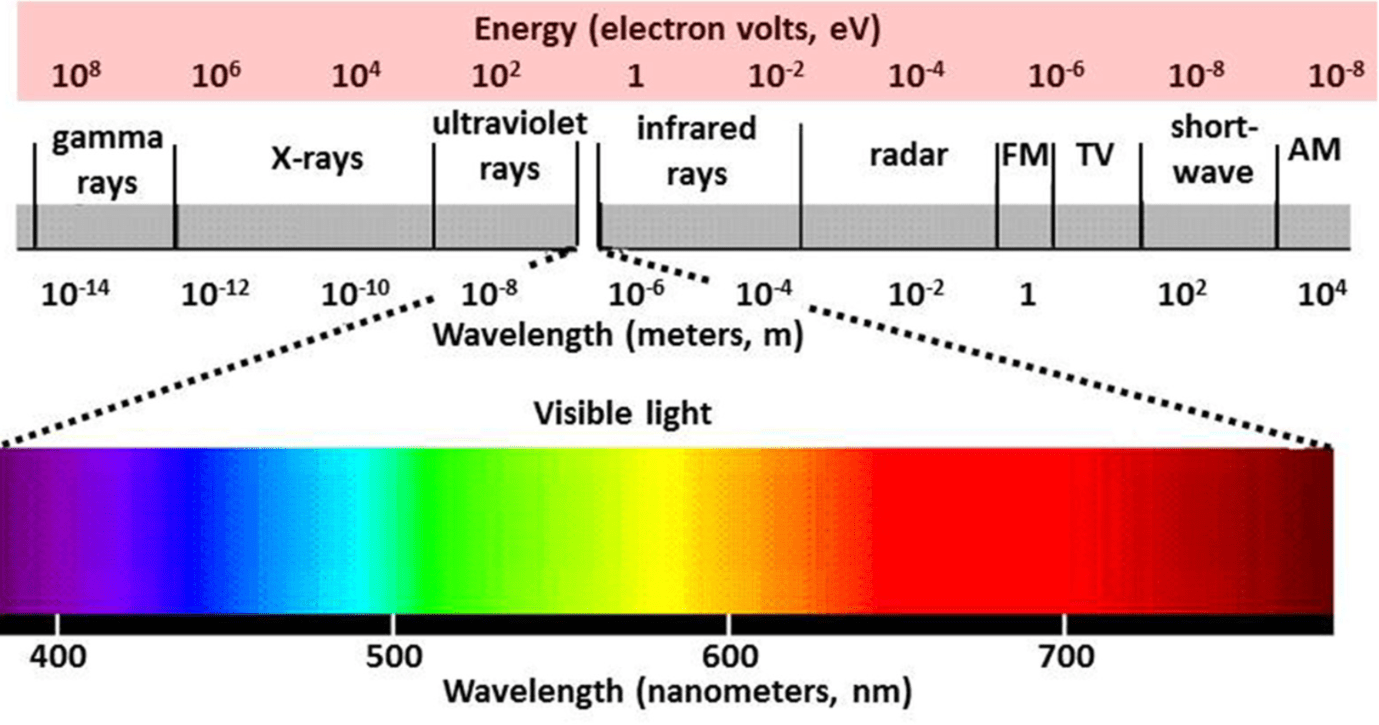
List the Electromagnetic Radiation Spectrum starting from the shortest wavelength (include visible light, aka the rainbow)
Is blood a heterogeneous or homogeneous mixture?
Homogeneous
What is the molar mass of caffeine (C8H10N4O2)?
194.19 g/mol
What are the polyatomic elements?
P4, S8, Se8
Using compounds as an example, explain the laws of multiple proportions, conservation of mass, and the law of constant composition.
Law of Conservation of Mass: number of atoms in = number of atoms out (H2O + CO2 = H2CO3)
Law of Constant Composition: a compound will always be made of the same elements with the same mass percents
Law of Multiple Proportions: When two elements form more than one compound, the masses of element B that combine with a fixed mass of element A always combine in ratios of small whole numbers, demonstrating that atoms combine in fixed, simple ratios
The density of a certain substance is 6.3 g/cm3. Aliens have landed on your planet and demand that properties be expressed in their units of density, glorps/eeks3. 1 glorp = 0.324 g, and 1 cm = 2.7 eeks. What is the density in alien units?
0.988 glorps/eeks3
FM-95, an FM radio station, broadcasts at a frequency of 9.51 × 107 s−1 (95.1 MHz). What is the wavelength of these radio waves in meters?
3.15 m
Which of the following scenes depict pure substances?
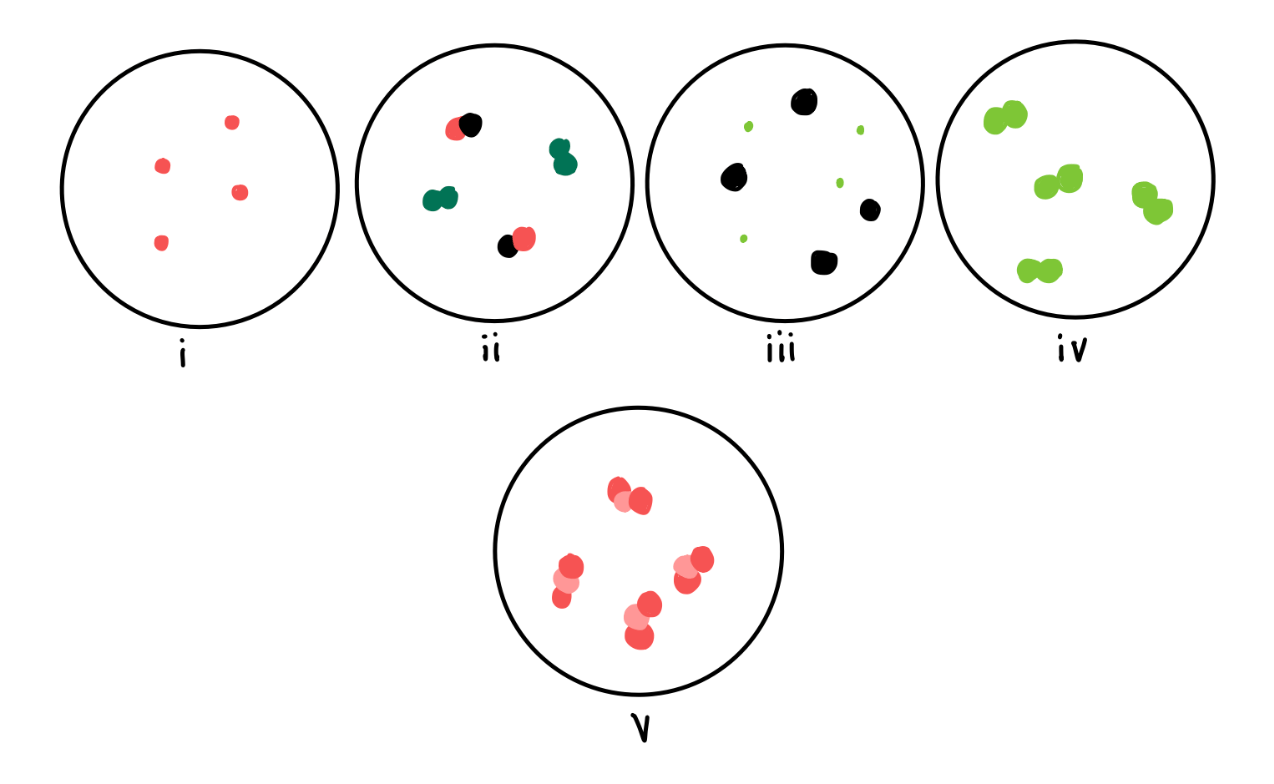
i, iv & v
Given 10.2 mol ethane, C2H6, what is the mass in grams?
3.1x102 g of C2H6
Describe the difference between accuracy and precision
Accuracy is how close the results are to the true answer, and precision is how close they are to one another
Yeast converts glucose to ethanol and carbon dioxide during anaerobic fermentation, as depicted in the simple chemical equation here:
glucose⟶ethanol+carbon dioxide
(a) If 200.0 g of glucose is fully converted, what will be the total mass of ethanol and carbon dioxide produced?
BONUS: What law does this represent?
200g
BONUS: Law of Conservation of Mass
The distance between the centers of the two oxygen atoms in an oxygen molecule is 1.21 × 10-9 dm. What is this distance in inches?
4.76x10-9 inches
A photon of light produced by a surgical laser has an energy of 3.027 × 10−19 J. Calculate the frequency and wavelength of the photon. What is the color of the emitted light?
ν = 4.568 × 1014 s−1; λ = 656.3 nm; red
Classify the six italicized properties in the following paragraph as chemical or physical:
Fluorine is a pale yellow gas that reacts with most substances. The free element melts at −220 °C and boils at −188 °C. Finely divided metals burn in fluorine with a bright flame. Nineteen grams of fluorine will react with 1.0 gram of hydrogen.
Pale yellow: physical
Gas: physical
Reacts with most substances: chemical
Melts at -220 oC: physical
Boils at -188 °C: physical
Metals burn in fluorine: chemical
Nineteen grams of fluorine will react with 1.0 gram of hydrogen: chemicals
How many molecules of fructose, C6H12O6, are in a sample of 53.02 g?
1.772 x 1023 molecules
Is the following a scientific hypothesis, a theory, or a law: The sun is made of children’s lost teeth
Hypothesis
Calculate the average atomic mass of Lead (Pb) given the mass and abundance of its 4 isotopes: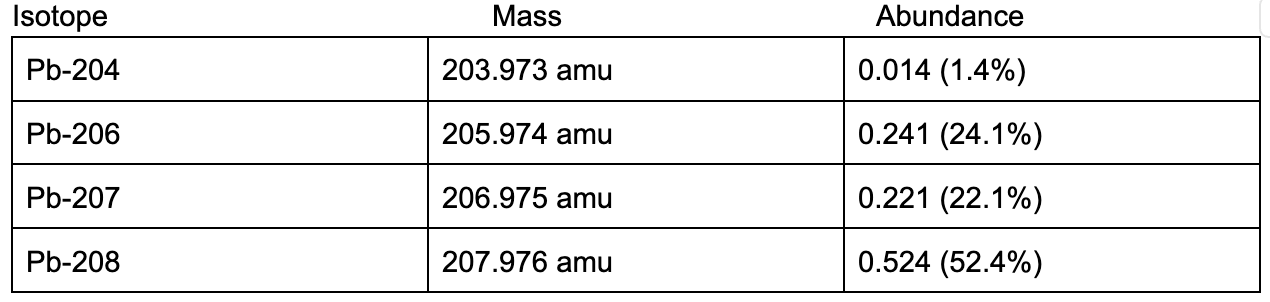
207 amu (should be correct sig figs; full answer = 207.216255)
The recommended dose for ibuprofen in an infant is 12.5 mg/kg of body mass every four hours. If ibuprofen is in liquid form with a density of 160 ng/5.0 dL how many mL should be administered to an infant weighing 8.9 lbs (1 kg= 2.2 lbs)
1.6x108 mL
What is the threshold frequency for sodium metal if a photon with frequency 6.66 × 1014 s-1 ejects an electron with 7.74 × 10-20 J kinetic energy? Will the photoelectric effect be observed if sodium is exposed to orange light?
5.49 × 1014 s−1; no
When elemental iron corrodes, it combines with oxygen in the air to ultimately form red-brown iron(III) oxide called rust.
If a shiny iron nail with an initial mass of 23.2 g is weighed after rusting, would you expect the mass to have increased, decreased, or remained the same? Explain
BONUS: Is this a physical or chemical change?
Increased as it would have combined with oxygen in the air, thus increasing the amount of matter and therefore the mass.
BONUS: Chemical
A certain nut crunch cereal contains 11.0 grams of sugar (sucrose, C12H22O11) per serving size of 60.0 grams. How many servings of this cereal must be eaten to consume 0.0278 moles of sugar?
0.865 servings, or about 1 serving
When and where is your exam?
7pm on Tuesday the 27th
location:
Last names start with A – G: Colombia 150
Last names start with H – L: Willamette 100
Last names start with M – P: Lillis 282
Last names start with Q – Z: Lillis 182