True or False
Some elements, such as copper, tin, gold, and silver, have been known since antiquity.
True
The horizontal rows on a periodic table are called _____.
periods
The period in which an element resides indicates the ______ of that element.
valence shell
In the 1860s, the elements were organized into rows in order of increasing _____.
mass
The vertical columns on a periodic table are called ____.
groups
The valence shell is the _____.
outermost energy level
Considered to be the father of the periodic table.
Dmitri Mendeleev

Groups 1,2, and 13-18 are known as the ______.
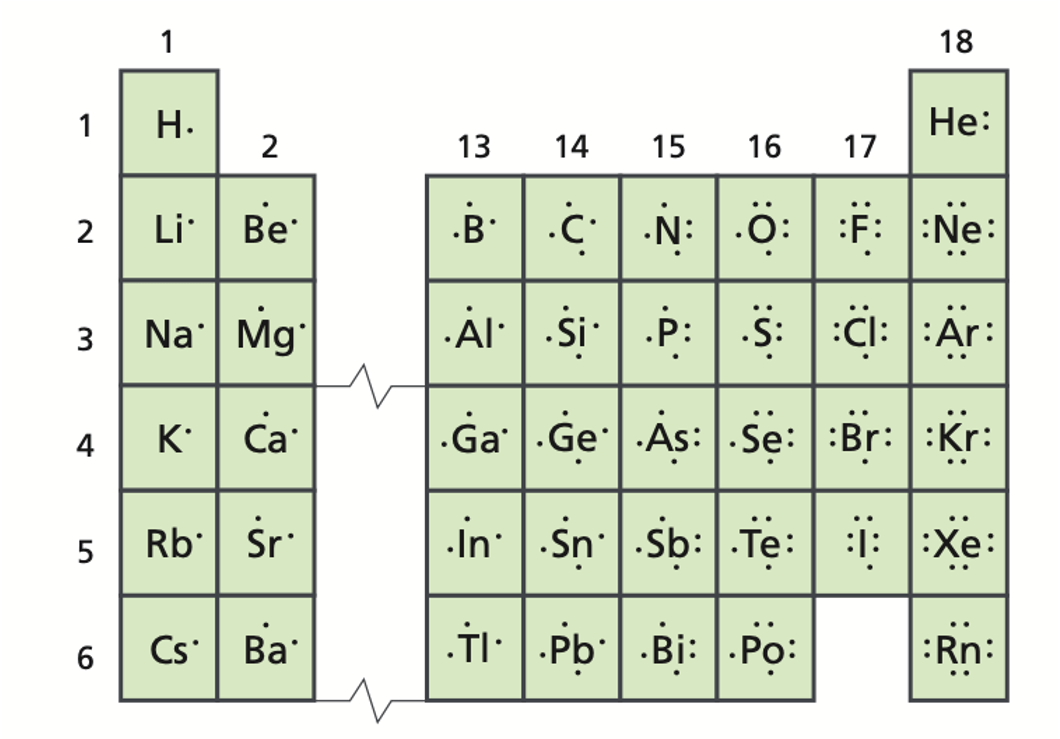
representative elements
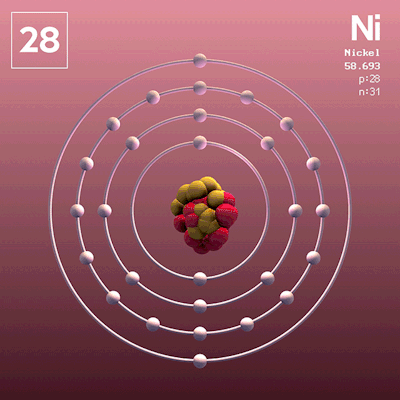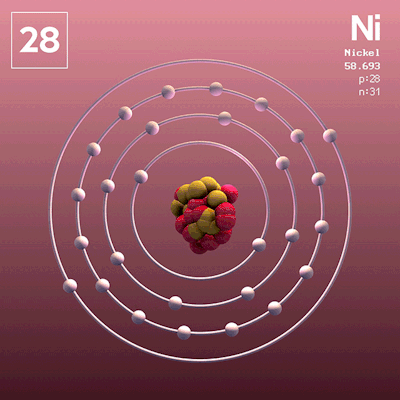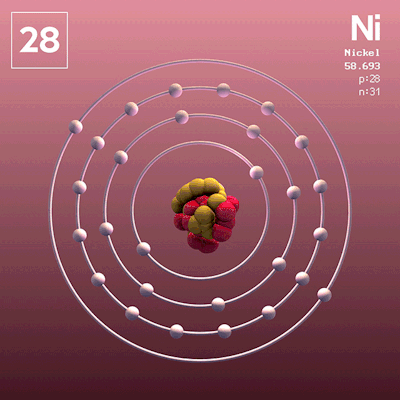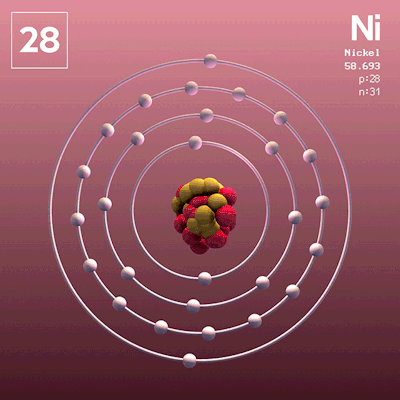
The group number indicates the number of _____ in the valence shell.
valence electrons
This scientist was actually the first to organize the elements into a table.
John Newlands
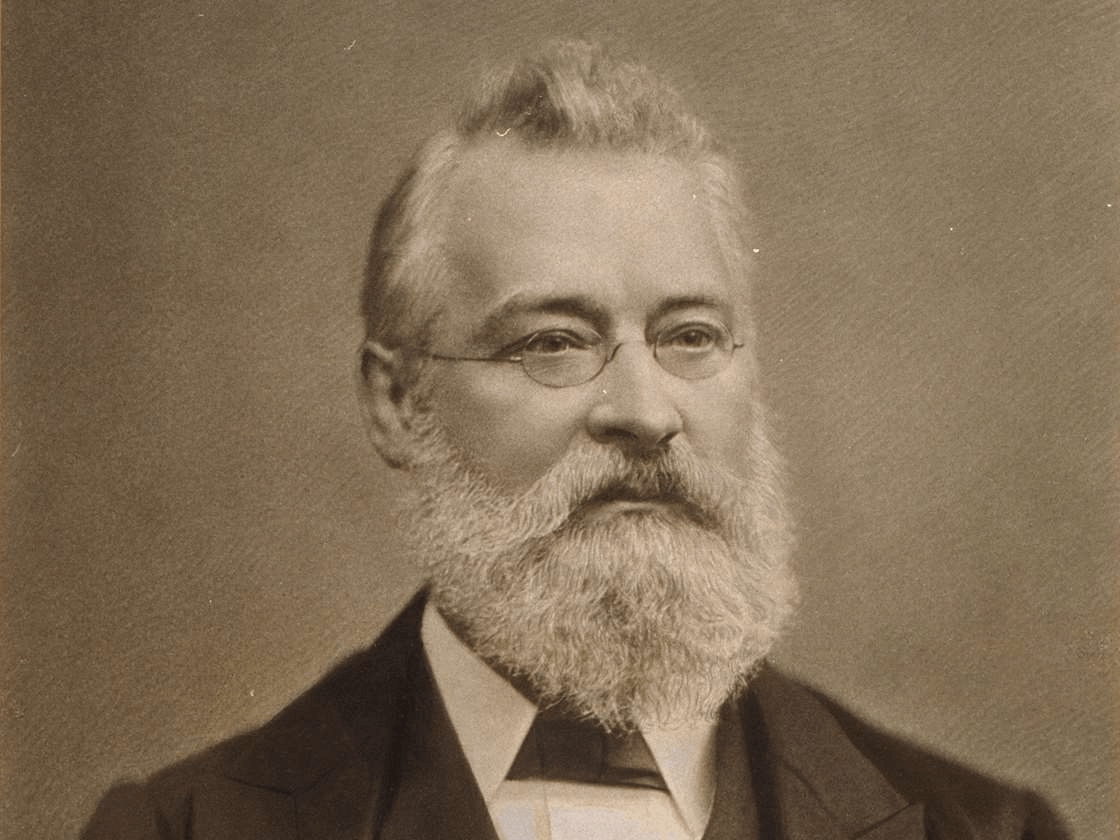
What are the three broad categories of elements on the periodic table?
Metals, Semimetals, and Nonmetals
How many valence electrons does element 6 (carbon,C) have?
four
In 1913, this scientist reorganized the elements on the periodic table using a parameter that is still in use today rather than atomic mass.
Henry Moseley
Name three properties of metals
good conductors, shiny, malleable, ductile
Element 6 (oxygen, O) has it's valence electrons in what shell? Give a number.
2nd shell/energy level
True or False
Mendeleev was able to predict the ____ of elements that had yet to be discovered.
properties
Mostly mostly comprised of gases.
nonmetals
Group 18 elements are called the ____.
nobel gases
____ are pure substances that cannot be further divided into separate substances.
elements
An element that is a dull in appearance, a poor conductor, and also brittle is likely a member of what broad category of elements?
nonmetals
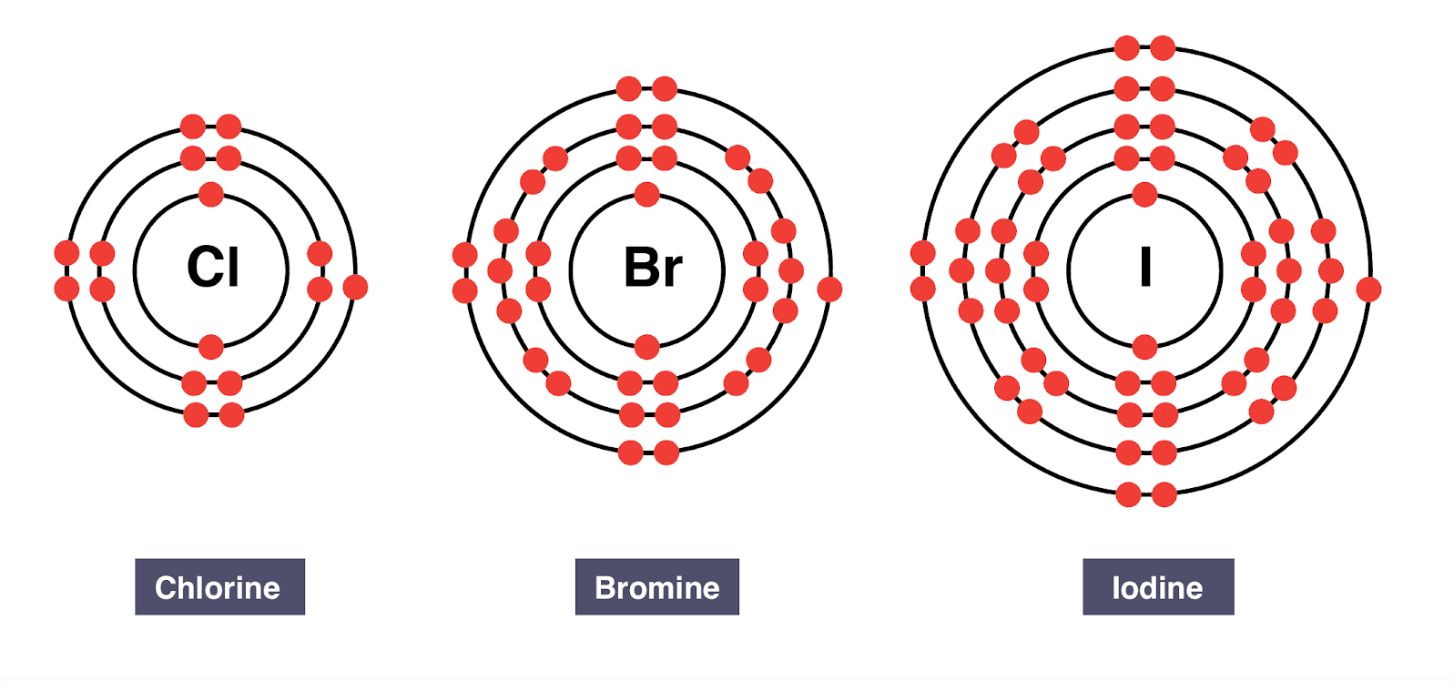
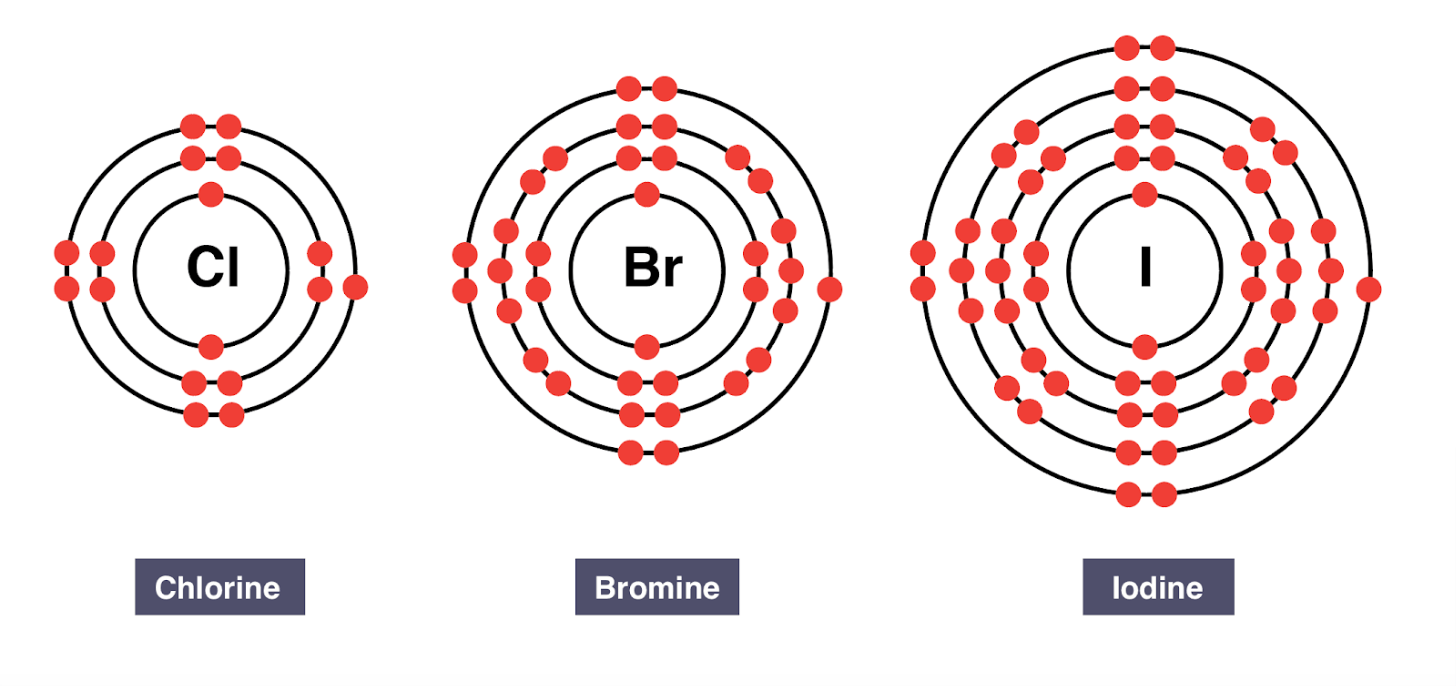
Group 17 elements are called the ____
halogens
Elements on the modern periodic table are arranged in order of increasing ____.
atomic number/number of protons
Elements that are generally shiny, brittle, and semiconductors are called _____.
semimetals/metalloids
Magnesium belongs to what group?
alkaline earth metals
A ___ is an arrangement of elements in columns, based on a set of properties that repeat from row to row.
periodic table
Germanium (Ge) is a _____.
semimetal
How many valence electrons do the alkali metals have?
one valence electron
The periodic repetition of properties is called the _____.
periodic law
Uranium is a _____.
metal
All halogens have ____ valence electrons.
seven valence electrons