He was the first to discover that when elements are arranged in order of their atomic masses their properties repeat every 8th element.
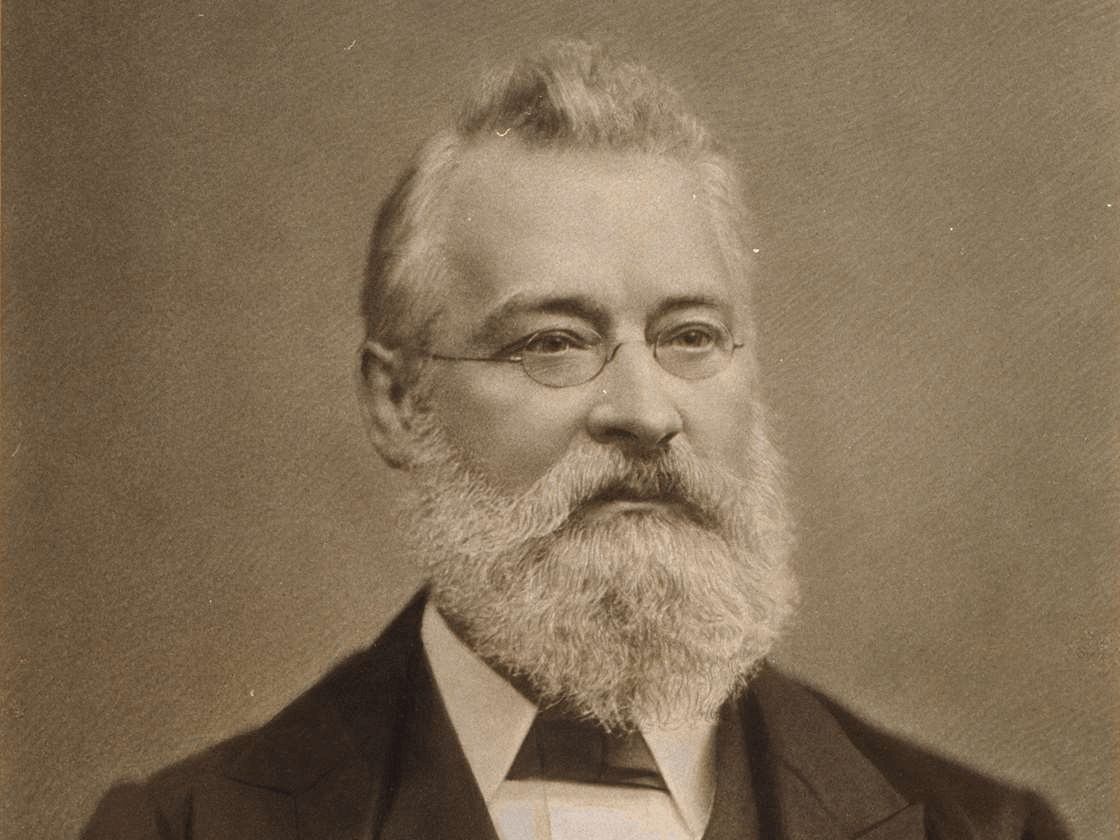
John Newlands
Elements are generally divided into what 3 categories?
metals
nonmetals
semimetals/metalloids
Like charges ___ and opposite charges ___.
repel, attract
What is the trend for atomic and ionic radius on the periodic table?
left, down
What is the trend for ionization energy on the periodic table.
Ionization energy increases up and to the right
Electronegativity increases as you move to the ___ and ___.
right, up
Considered the "father" of the periodic table

Dmetri Mendeleev
These elements are shiny, malleable, ductile, and good conductors of heat/electricity.
metals
Within an atom, the electrons are attracted to the ___.
nucleus/protons
What causes the vertical trend in radius on the periodic table?
more energy shells as you move down a group.
What is ionization energy?
Energy required to remove electron from valence shell.
What is electronegativity?
The ability of an atom's nucleus to attract electrons from another atom.
Figured out that elements must be arranged by atomic number rather than atomic mass.

Henry Moseley
Iodine (I), chlorine (Cl), and bromine (Br) belong to which group? Give group name.
halogens
As atomic number increases, what happens to the charge of the nucleus? Be specific.
charge becomes more positive
Which atom has the smallest radius?
A. Te, tellurium
B. Se, selenium
C. S, sulfur
D. O, oxygen
D. O, oxygen
Which atom has the lowest ionization energy?
A. Te, tellurium
B. Se, selenium
C. S, sulfur
D. O, oxygen
A. Te, tellurium
What is the most electronegative element?
Fluorine
Proposed the first quantum model of the atom, depicting the electrons orbiting the nucleus like planets around the sun.

Niels Bohr
Lithium (Li), sodium (Na), and potassium (K) belong to which group? Give group name.
Alkali metals
If you have two charged particles and the magnitude of one increases, what happens to the electric force between them?
electric force increases
F = k q1q2/r2
Why does atomic radius decrease as you move across a period (left to right)?
Increase in protons/atomic number/ nucleus' pull on electron cloud.
Which element has the highest ionization energy?
A. C, carbon
B. N, nitrogen
C. O, oxygen
D. F, fluorine
D. F, fluorine
Which atom has the lowest electronegativity?
A. Te, tellurium
B. Se, selenium
C. S, sulfur
D. O, oxygen
A. Te, tellurium
Discovered equations to describe how electrons are arranged around the nucleus in volumes of space called orbitals.
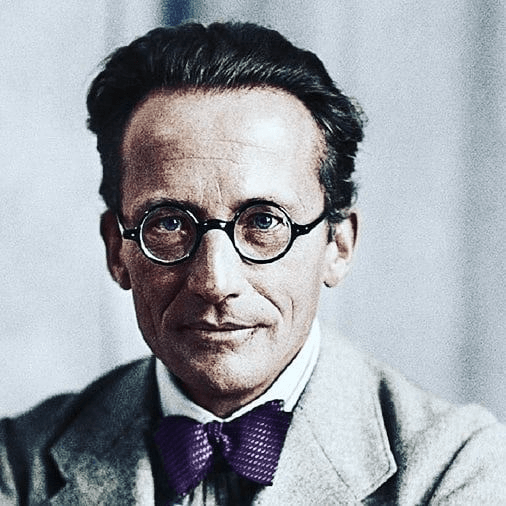
Erwin Schrödinger
Beryllium (Be), calcium (Ca), and magnesium (Mg) belong to which group? Give group name.
Alkaline earth metals
If you have two charged particles and the distance between them increases, what happens to the electric force between them?
the electric force decreases
F = k q1q2/r2
Which particle has the largest radius?
A. Cu
B. Cu+
C. Cu2+
D. Cu3+
A. Cu
Why is it easier to remove the first electron than it is to remove the 2nd or 3rd electron from an atom?
As the radius decreases, the pull on electrons by nucleus increases.
Which element has the highest electronegativity?
A. C, carbon
B. N, nitrogen
C. O, oxygen
D. F, fluorine
D. F, fluorine
Discovered the nucleus
Rutherford
Why are noble gases generally unreactive?
octet/full valence shell/orbitals in valence shell are filled
Only valence electrons are involved in chemical reactions. Why?
loosely held by the nucleus due to weaker electric force.
Which particle has the largest radius?
A. Cl
B. Cl-
C. Br
D. Br-
D. Br-
Which element has the LOWEST ionization energy?
Cl-, chloride
Cl, chlorine
O2-, oxide
O, oxygen
F-, fluoride
Br-, bromide
Br, bromine
Br, bromine
Why does electronegativity increase?
stronger attractive force from nucleus