Matter with definite shape and volume.
What is solid?
A change in some properties of matter that does not change what the substance is made of.
What is physical change?
A process in which a new kind of matter forms.
What is chemical change?
A substance where different materials are put together but keep its own properties.
What is mixture?
In a water and sugar solution, what is the sugar?
What is the solute?
Matter that does not have a definite shape or volume.
What is gas?
100 degrees celsius (water)
What is the boiling point of water?
The scientific law that in any physical or chemical change, the total mass of the matter does not change.
What is the law of conservation of matter?
A mixture in which the substances are evenly spread out and do not settle to the bottom of the container.
What is a solution?
To identify the differences between two or more objects.
What is to differentiate?

What is liquid?
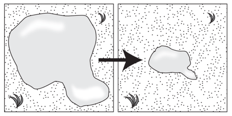 This is evidence of what physical change?
This is evidence of what physical change?
What is evaporation?
True or False: In a chemical change, a new substance forms and the substance has different properties than the original substance.
True
What happens with the particles of both substances when sugar dissolves in water?
The particles of both substances become evenly spread out.
The substance in which the solute (salt) is being dissolved in a salt and water solution.
What is solvent?
True or False: The mass of a liquid substance is the same as the mass of the substance in solid form.
True
True or False: Rusting of a metal is a physical change.
False, rust is evidence of a chemical change.
Explain where you saw a chemical change in the balloon lab.
The baking soda reacted with the vinegar to form a new substance, a gas, which was evidence of a chemical change.
One student opens a jar of iron filings and sand. Another student holds a magnet over the sand and iron filings. What is the purpose of this procedure?
To separate the mixture.
 What would the model look like if a chemical reaction had occurred?
What would the model look like if a chemical reaction had occurred?
Mr. Stokes is going to pour the liquid substance from the container shown into a large bucket. Which observation will Mr. Stokes most likely make? (Tell about shape and volume.)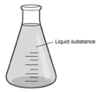
The liquid will spread and take the shape of the container. The liquid will fill the bottom of the bucket, while the volume of the liquid will remain the same.
Why do railroads only warp when they get hot and not cold?
When the tracks get hot, the metal expands and causes the tracks to push up against other sections. When they get cold, they shrink and pull away from one another instead of pushing against one another.
Explain what happened with the penny over time when it was placed on top of the vinegar. What type of reaction occurred and how do you know? What was the evidence.
Over time, the copper penny started to turn green. The copper metal that makes up a penny combined with oxygen in the air to form a green substance. The green substance is copper oxide. Evidence of a "formation of a solid" and "color change" proves there was a chemical change.
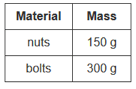 If the nuts and bolts were mixed together, what would be the mass of the mixture?
If the nuts and bolts were mixed together, what would be the mass of the mixture?
The mass is 450 g. The total mass of the mixture is equal to the total mass of each of the components.
An inflated balloon was placed in a freezer and checked on several hours later. When it was checked on, the balloon had changed shape. Explain why the balloon changed shape after being in the freezer?
The cold air in the freezer caused the volume of the air in the balloon to change.