PBr3
Phosphorus tribromide
The two types of atoms that form covalent bonds
Nonmetal and nonmetal
The number of electron pairs shared in a double bond
two
In an ionic bond, the metal _________ its electron(s) to the nonmetal.
Transfers
The valence number and group name of this element
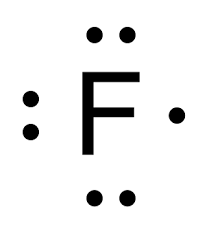
Seven (7), Halogens
HCl
Hydrogen monochloride
Valence electrons are ___________ in a covalent bond.
Shared
Name ONE property of covalent compounds.
Low melting and boiling points, flammable, poor conductors of heat and electricity
The shorthand electron configuration for scandium
[Ar] 4s2 3d1
ONE reason this Lewis structure incorrect?
Hydrogen can only make ONE bond.
Carbon can make FOUR bonds.
Oxygen can make TWO bonds.
S2F7
Disulfur heptafluoride
The number of covalent bonds that phosphorus would form
Three
H (2.1) and Se (2.4)
Polar, Nonpolar or Ionic
Nonpolar; 2.4-2.1 = 0.3 (less than 0.4)
Name an element with similar properties to calcium
Be, Mg, Sr, Ba, or Ra
H2, N2, F2, O2, I2, Cl2, Br2
N2O
Dinitrogen monoxide
The reason atoms form bonds with other atoms
Octet rule; stability; be like noble gases
The meaning of the HONC rule
Hydrogen and Halogens (1 bond), Oxygen (2 bonds), Nitrogen (3 bonds), Carbon (4 bonds)
Oxygen gas forms a ___________ bond.
Double
The name of Cl2
chlorine gas or chlorine
C4H8
Tetracarbon Octahydride
Which of these is a/are covalent compound(s)?
HBr, CaCl2, NO, CuCl3, PbF2
HBr, NO
The difference between a polar and nonpolar covalent bond
Polar (atoms share electrons UNEQUALLY). Nonpolar (atoms share electrons EQUALLY)
The number of unshared pairs in the Lewis dot structure for CH3F
The diatomic element that forms a triple bond
N2