This is the branch of chemistry with an area of focus relating to most carbon-containing chemicals.
What is Organic Chemistry
Give the place value of the bold digit below:
1376.791
What is ones place
An alloy of steel contains 17.5 grams of carbon (C) and 52.5 grams of iron (Fe). What is the percentage of carbon in the alloy.
What is 0.25%
Rewrite the following equation for x:
8x − 2 = −9 + 7x
What is x = -7
What is being measured on the vertical axis?
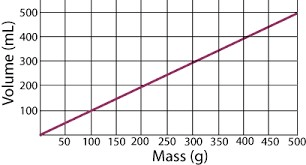
What is Volume in mL
A substance that possesses the same composition and properties wherever it is found.
What is a chemical
Round 27.421 to the nearest ones place.
What is 27
On the first chemistry test, 12 students got As, 18 students got Bs, and 20 students got Cs. What percentage of students scored 80 or higher?
What is 60%
Rewrite the following equation for h:
h − 1 = 5h + 3h − 8
What is h = 1
What is the range of the horizontal axis?

What is 0g to 500g
This is the step in the scientific process where a tentative explanation is given for what is being observed. Normally written as an "If, Then" (because) statement.
What is a Hypothesis
Round 5.13574 to the nearest hundredth place.
What is 5.14
5.0 mL of hydrochloric acid (HCl) is diluted to 100 mL using deionized water. What is the percent concentration of hydrochloric acid?
What is 5%
Density is the amount of mass in a given volume. The formula for density is D = m/v. Rewrite this equation for volume (v).
What is V = m/D
Describe the relationship between mass and volume according to this graph.

As mass increases, the volume increases
Label the following as an observation, hypothesis, experiment, or conclusion:
"It is worth noting that students achieve high test scores when they consistently do their homework."
What is an Observation
a block of Osmium (the densest metal on earth) measures 3.5 in. by 3.5 in by 6.8 in. Calculate its volume to the nearest hundredth
What is 83.30 cubic inches
90 mL of hydrogen peroxide is mixed with enough water to make 3000 mL of solution. What percentage of the solution is hydrogen peroxide?
What is 3%
The equation for converting Celsius to Kelvin (K) is
K = (° C + 273.15). What is 296.56 K in ° C?
What is 23.41° C
Approximately what is the volume of the substance at 350g?

What is 350 mL
If experimental results do not support your hypothesis, you should:
a. Do more experiments
b. Modify your hypothesis
c. Write a conclusion
b. Modify your hypothesis
A solution's temperature drops 57 degrees from 22.5 degrees Celsius. What is the final temperature?
What is -34.5 degrees Celsius
25 mL of ethanol is added to enough water to produce 200. mL of the solution (mixture). What percent of the total solution is ethanol.
What is 12.5%
Magma burns at a temperature of 2000° F. Use the following equations to convert this temperature from Fahrenheit to Kelvin. Round to the nearest ones.
K = (T°C + 273.15)
T°F = (1.8)T°C + 32
What is 1366.48 K
Density = mass/volume (D=m/v). Use the list of densities below to identify the most likely substance in the line graph.


What is water?