a multiple of an empirical formula showing the number and type of atoms in a molecule
What is molecular formula
How many atoms are in 1.85 mol Pb?
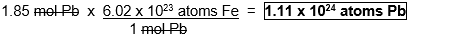
If the ratio of carbon to hydrogen atoms is 1:4, what is the chemical formula?
What is CH4
What is the molar mass of chlorine gas?
What is 70.9 g/mol
What units are molar mass in?
What are grams per mole (g/mol) or amu (atomic mass units)
The percent by mass of each element in a compound is...
What is percent composition
How many atoms are in 1.5 moles of copper?
What is 9.03x1023 atoms of Cu
Find the percent composition of magnesium in magnesium chloride
What is 25.5% Mg in MgCl2
What is the molar mass of C6H12O6?
What is 180.2 g/mol
Can the empirical and molecular formulas ever be the same?
Yes!
What is the SI unit to measure the amount of a substance?
What is the mole
How many moles are in 25.0 grams of sodium chloride?
What is 0.428 moles NaCl
If the empirical formula is CH and the molecular molar mass is 39, what is the molecular formula?
What is C3H3
What is the percent composition of Ba in Ba(OH)2?
What is 80.1%
Name two of the seven diatomic molecules.
What are: hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine
What is the empirical formula?
What is the smallest whole number ratio of the atoms in a compound
How many molecules are in 120. g H2O2? (MM = 34.02 g/mol)
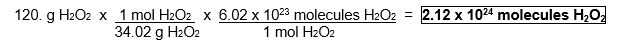
If the empirical formula is CH2O and the molecular molar mass is 30, what is the molecular formula?
What is CH2O
What is the percent composition of nitrogen if the molecule contains 5.46g of nitrogen and 3.64g of oxygen?
What is 60.0%
How much iron in a 34.6g sample of Fe2O3?
% Fe=70%, so there is 24.2g of Fe in the sample
What is one mole?
What is the 6.02x1023 number of particles
Convert 4.03 x 1022 molecules of water to moles of water.
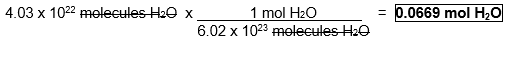
Find the empirical formula of a compound containing 7.8% carbon and 92.2% chlorine
What is CCl4
How many hydrogen atoms are in 5 molecules of H2O?
What are 10 atoms
The molar mass of a gas is 71g. What is the density at STP?
What is 3.17g/L