Basic building block of matter.
This part of an atom has a negative charge.
What is solid, liquid and gases
Materials in this can be separated?
What is a mixture?
These are located in the nucleus and list its charges.
What are the proton (+) and Neutrons (no charge)
Items that sink in water have a density that is _____________ .
What is less than water or less than 1g/cm3 ?
There are ____ different types of atoms in a molecule of sugar (C6H12O6). And what are their names
What is 3, Carbon, Hydrogen and Oxygen?
This is happening to the image.
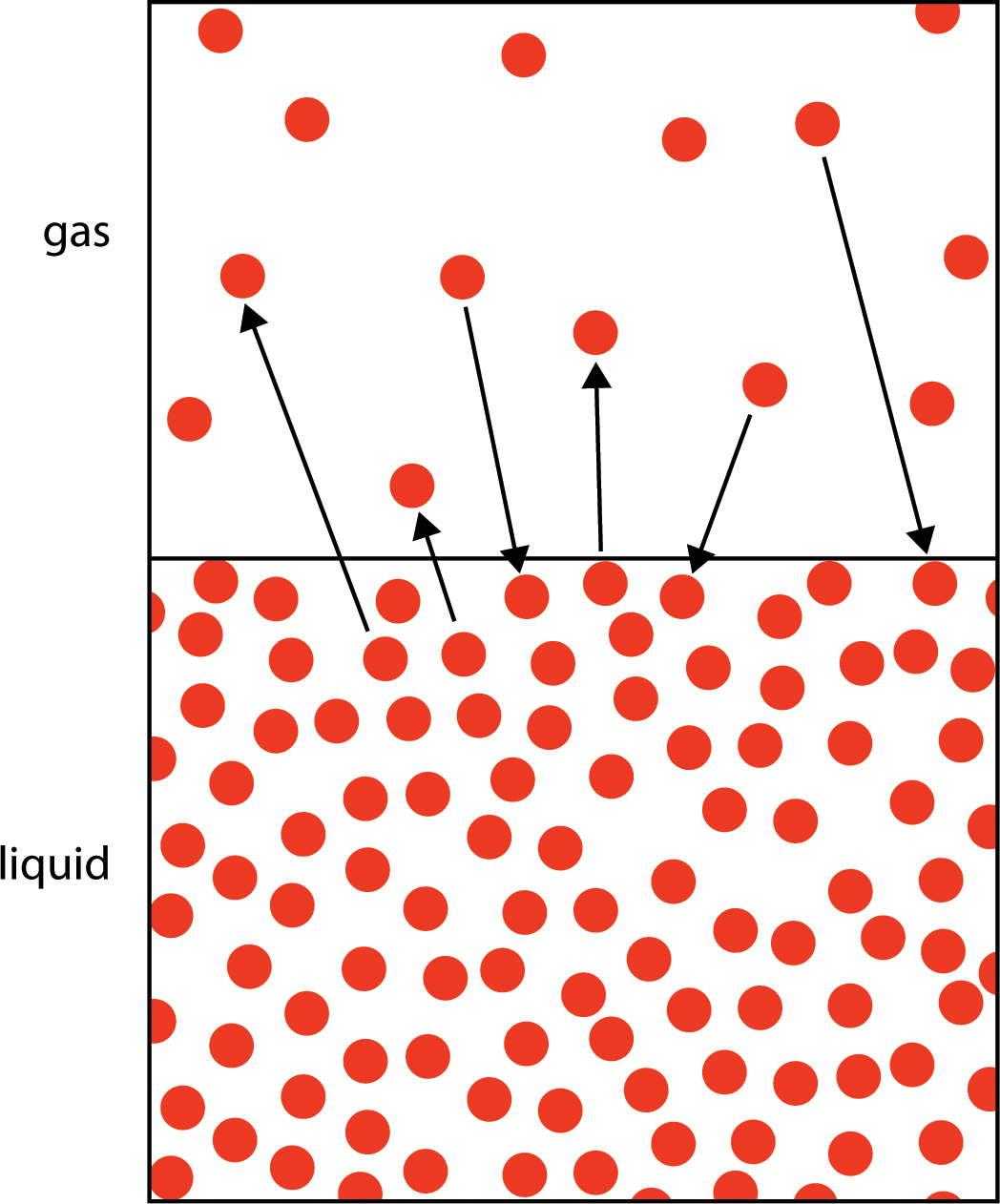
What is warming up and evaporation
This is the name for which a solute gets disolved in a solution.
What is a solvent?
If more solute is added to a _________ solution, it will not dissolve.
What is saturated?
List at least 2 property of Metals.
What is shiny, conducts heat and electricy well, and bendable?
This element shares similar properties to Calcium.

What is anything in the same column.
This happens to atoms as matter gets colder.
What is the atoms slow down and get closer together?
True or False (if false, correct to make the statement true):
We can only disolve solids in a solution.
False, solids, liquids and gases can be disolved into a solution.
Define a Compound
Decribe the mass of an object on the moon and earth.
What is they will be the same because mass is how much matter something has and it doesn't change?
this is how many levels or "highways" are there and how many electrons can each level hold.
What is 3?
level 1 - 2
level 2 - 8
level 3 - 18
List all the "points" and define each one

This is the major difference between pure metals and alloys? List 2 examples of pure metals and 2 examples of alloys
What is that pure metals are made up of only 1 element and alloys are a mixture of 2 or more metals.
Pure: gold, silver, aluminum, iron, nickle.
Alloy: Steel, Bronze, Brass
They are similar because they both descibe how saturated a solution is. They are opposites, Concentrated means there is alot of solute and diluted means little solute and not very saturated.
Calculate the density of an object that has a mass of 30g and has a length of 3cm, width of 2cm and height of 2cm
What is 2.5 g/cm3.
Volume = l x wx h D = M/V
= 3*2*2 = 12cm3
30g/12cm3 = 2.5
Decribe what happens and an atoms properties when they are combined and 1 example
What is they change? For example, Salt NaCl, Na sodium is an explosive metal, and Cl is a poisionous gas but when combined, we can safely eat it.
Condensation on a can is the process of water vapor turning into liquid water droplets on the surface of a cold can. This happens when water vapor in the air comes into contact with the can's cold surface.

Compare and contrast a mixture and a solution and give 1 example of each.
They are similar because a solution is a type of mixture. They are different because in a mixture, things are easily separted, in a solution things are mixed evenly throughout.
