The formula used to calculate average reaction rate.
change in[concentration]/change in [time]?
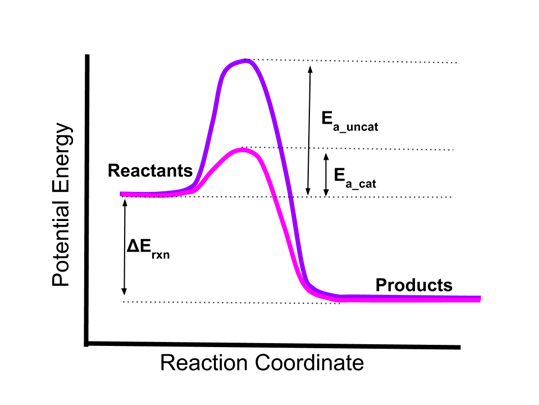 Which of the 6 factors do you think is affecting this reaction?
Which of the 6 factors do you think is affecting this reaction?
Catalysts
What state of matter are all precipitates?
Solid
What, on the molecular level, causes chemical reactions occur in the first place.
Collision!
Why does temperature affect reaction rate?
It changes the kinetic energy of that substance, resulting in a faster or slower collisions.
light - photosynthesis
What is the difference between NaCl (aq) and NaCl (L)?
(aq) is salt dissolved in water, (L) is pure liquid (molten) salt
Why does concentration affect reaction rate?
This alters the amount of collisions taking place within a reaction.
Increasing pressure can increase the rate of reactions for what kind of substances?
Gas
What is an isotope?
What is the required activation energy for this reaction?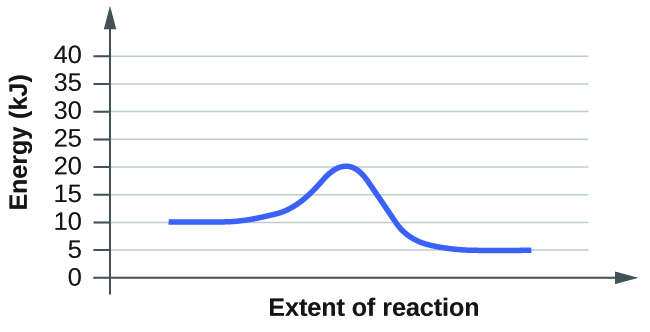
10 kJ
A substance that increases the speed of a chemical reaction.
What is an catalyst?
Decreasing volume of a gas will change this factor.
Pressure
What element has the highest electronegativity?
Fluorine
Think about activation energy... now think about how ammonia is made: combining hydrogen and nitrogen. Pretend you add hydrogen and nitrogen to a container to make ammonia. In terms of activation energy, why might the ammonia unbond to form hydrogen and nitrogen atoms again in that container.
The activation energy for the breakdown of ammonia is equal to that of creating ammonia.
What metal is a liquid a room temperature?