When written in scientific notation the number 0.000053 is
5.3 x 10-5
How many significant figures are in 203.0
4
57.2 g is equivalent to how many kg?
0.0572 kg
In the number 0.3425, which is the last certain digit?
2
Of meters, liters, seconds, and Kelvins, which is a derived unit?
liters
When written in standard notation, 6.22 x 1010 is
6.2200000000
How many significant figures are in 0.00350
3
975 oC is what temperature in Kelvin?
1248 K
In the number 1530, which is the uncertain digit?
3
If a series of results are similar in value but far from the expected value they are said to be
precise but not accurate
In scientific notation the sign of the power of ten for small numbers is
negative
What two types of numbers have unlimited significant figures?
Numbers from counting
Numbers that are defined
48 mile/hr is how many ft/s?
70 ft/s
Three students measure the mass of a 5.00 g object and obtain the following masses:
5.00 g 5.03 g 5.10 g
How many of the measurements are correct?
2
The desity of a substance is 0.92 g/mL. What is the volume in mL of 50. g of the substance?
54 mL
The result of
8.7 x 1013 X 5.5 x 105 is
4.8 x 1019
The result of 33200 divided by 12.00 is
2770
2.5 x 106 ns is how many ms?
2.5 ms
What is the volume in the following image?
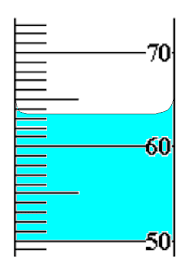
Between 63.1 and 63.9
A calculator display reads 0.0001376516642
When rounded to four significant figures, the number is
0.0001377
The result of
9.9 x 10-7 / 3.0 x 1011 is
3.3 x 10-18
The result of 2.77 + 1.7 x 10-2 is
2.79
12.01 g of carbon contains 6.02 x 1023 carbon atoms. What is the mass of 1.89 x 1025 carbon atoms?
377 g
Wheen 50.1 mL of water is combined with 1.75 mL of water, what is the numerical value of the uncertain digit in the combined volume?
9
Who is the greatest cat in the history of Chemistry?
Otis