What happens to molecules when something cools down?
The molecules move slower
Engineers design computers to cool down quickly so they do not break. What happens to the molecules in a computer when the temperature of the computer decreases?
The energy of the molecules in the computer decreases.
Eric pours some iced tea into a glass. When he puts the iced tea in the glass, the energy of the glass decreases. What must be true of the energy of the iced tea?
Its energy increases while the energy of the glass decreases
Which sample would transfer energy?
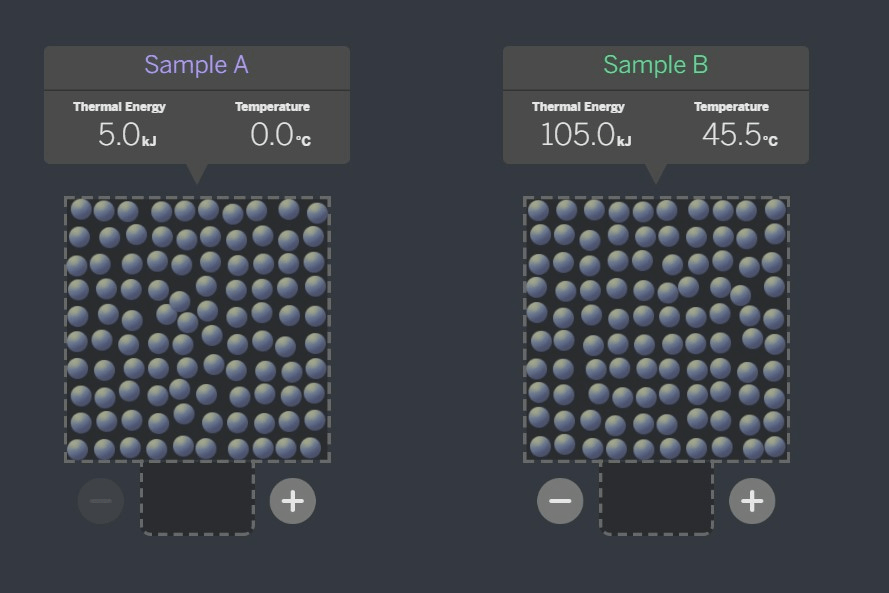
Sample B to Sample A
Energy between two objects transer until their temperatures are equal and they reach a stable state of ________.
equilibrium
This fast-food chain’s slogan is "I'm Lovin' It."
What is McDonald's?
What happens to molecules when something heats up?
The molecules move faster
Joseph puts a piece of toast on a plate. The toast is hot from the toaster. What is happening to the energy of the toast and the plate?
The toasts energy decreases while the energy of the plate increases
Action: you jumped into a frozen lake.
Model what would happen to your body temperature.
Body temperature would decrease.
Which way is energy transfering?

Sample A to Sample B
When things get _____, its molecules move faster and have faster kinetic energy.
hotter
This tech company is known for its logo featuring an apple with a bite taken out of it.
What is Apple?

Between Time 1 and Time 2, the sample....
a. got hotter
b. got colder
c. stayed the same
b. got colder
Rico and Bree are camping and have potatoes wrapped in foil to eat. Bree put her potato in the campfire for a while, but Rico did not. Now Bree’s potato has a high temperature. What is the difference between the molecules of the two potatoes?
The molecules of Bree’s potato are moving faster than the molecules of Rico’s potato.
Action: You left your cold drink outside on a hot summer day.
Model what would happen to your cold drink.
The drink would increase in temperature.
Which way is energy transfering?
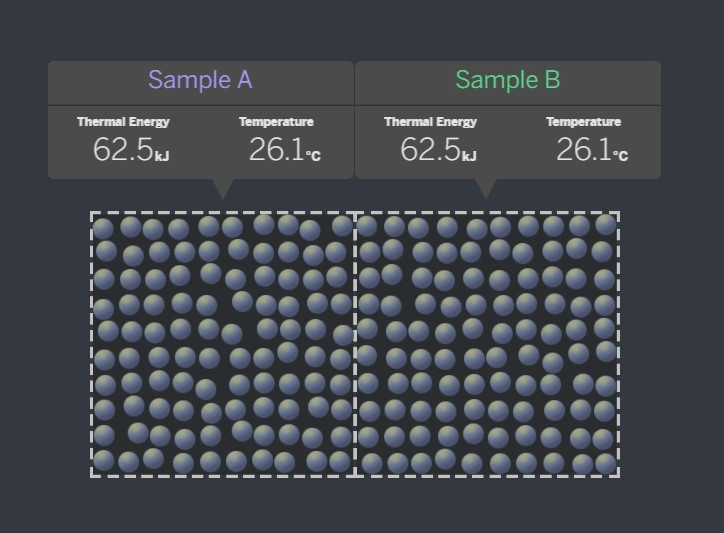
the samples have reached a stable state of equilibrium and are no longer transferring energy
When a thing gets _____, its molecules are moving slower and have less kinetic energy.
colder
This athletic brand’s slogan is “Just Do It” and its logo is a swoosh
What is Nike?
Action: You're eating a popsicle on a hot summer day.
What is happening to the temperature of the popsicle?
The temperaute of the popsicle is increasing
After a candle is blown out, the wax of the candle decreases in temperature. What happens to the molecules of the wax when the temperature of the wax decreases?
The energy of the molecules of the wax decreases.
Aki is playing cards and is about to put one card on top of another. Both cards are the same size and have the same number of molecules. The diagram above shows the cards now, before they touch each other.
How does the temperature of the top card compare with the temperature of the bottom card before the cards touch, and what will happen after the cards have been touching for a while?
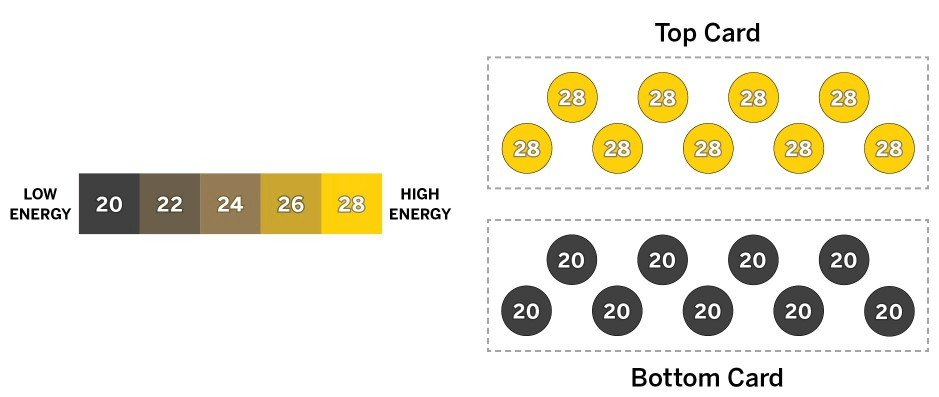
a. Before the cards touch, the top card is hotter than the bottom card. Once the cards are touching, the cooler bottom card will gain kinetic energy until both cards’ molecules have an energy of 28, because hotter things increase the temperature of cooler things.
b. Before the cards touch, the top card is hotter than the bottom card. Once the cards are touching, the top card will transfer kinetic energy to the molecules in the cooler bottom card until both cards reach the same temperature, which will be in between their starting temperatures.
b. Before the cards touch, the top card is hotter than the bottom card. Once the cards are touching, the top card will transfer kinetic energy to the molecules in the cooler bottom card until both cards reach the same temperature, which will be in between their starting temperatures.
An artist is using wooden cubes to build a statue. Yesterday, she placed one wooden cube on the statue, and today she is going to put an identical wooden cube on top of it. Both cubes are the same size and have the same number of molecules.
How does the temperature of the top cube compare with the temperature of the bottom cube now, and what will happen when the cubes have been touching for a while?
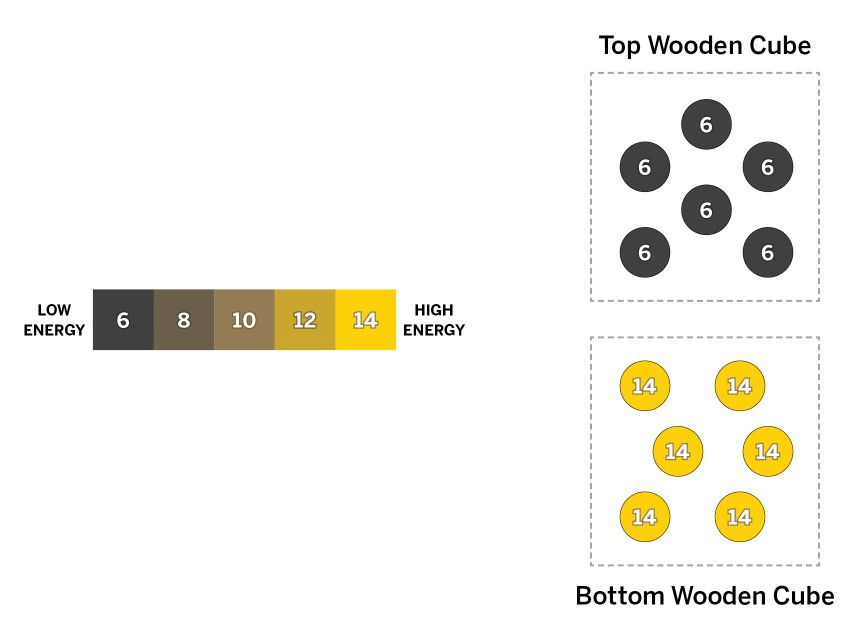
a. Before the cubes touch, the bottom cube is hotter than the top cube. Once the cubes are touching, the bottom cube will transfer kinetic energy to the molecules in the cooler top cube until both cubes reach the same temperature, which will be in between their starting temperatures.
b. Before the cubes touch, the two cubes are different temperatures. Once the cubes are touching, both kinetic energy and cold energy will transfer between the molecules in the two cubes until both cubes reach the same temperature, which will be in between their starting temperatures.
a. Before the cubes touch, the bottom cube is hotter than the top cube. Once the cubes are touching, the bottom cube will transfer kinetic energy to the molecules in the cooler top cube until both cubes reach the same temperature, which will be in between their starting temperatures.
When two things ______, their molecules transfer kinetic energy from the faster moving molecules to the slower moving molecules.
collide
This toy company’s slogan is “The Best Play on Earth.” It’s also known for its colorful plastic building blocks.
What is LEGO?
Action: You stacked a hot pancake on top of your cold pancake.
Using the Sim, model what would happen to the temperature of your cold pancake?
The temperature of the hot pancake would transfer to the cold pancake.
A chocolate maker is going to stack two bars of chocolate. The bars of chocolate are the same size and have the same number of molecules. The diagram above shows the chocolate bars now, before they touch each other. Use the information in the diagram to answer the question.
How does the temperature of the top chocolate bar compare with the temperature of the bottom chocolate bar now, and what will happen after the bars have been touching for a while?
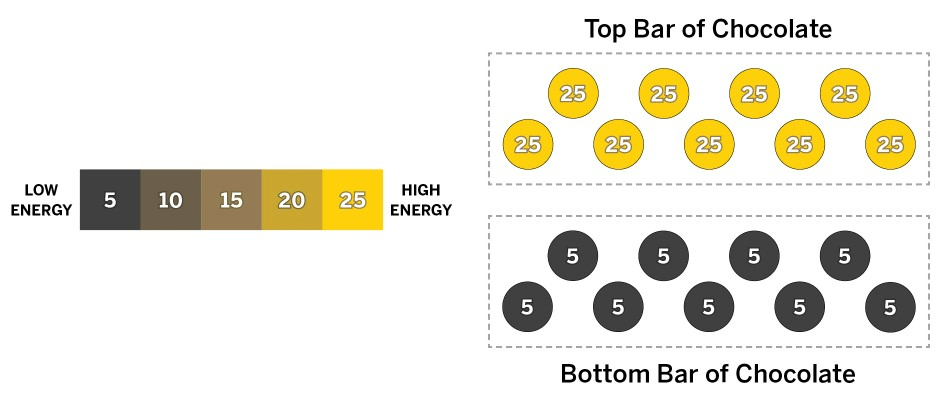
a. Before the bars touch, the top bar is cooler than the bottom bar. Once the bars are touching, the bottom bar will transfer kinetic energy to the molecules in the cooler top bar until both bars reach the same temperature, which will be in between their starting temperatures.
b. Before the bars touch, the top bar is hotter than the bottom bar. Once the bars are touching, the top bar will transfer kinetic energy to the molecules in the cooler bottom bar until both bars reach the same temperature, which will be in between their starting temperatures.
b. Before the bars touch, the top bar is hotter than the bottom bar. Once the bars are touching, the top bar will transfer kinetic energy to the molecules in the cooler bottom bar until both bars reach the same temperature, which will be in between their starting temperatures.
Claude is in the kitchen cooking and is going to stack one pan on top of another pan. The two pans are the same size and have the same number of molecules. The diagram above shows the pans now, before they touch each other.
How does the temperature of the top pan compare with the temperature of the bottom pan now, and what will happen after the pans have been touching for a while?
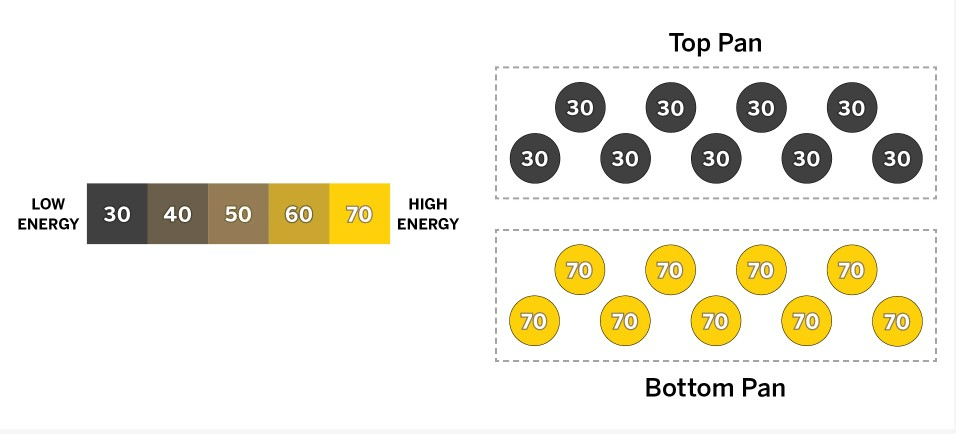
a. Before the pans touch, the bottom pan is hotter than the top pan. Once the pans are touching, the bottom pan will transfer kinetic energy to the molecules in the cooler top pan until both pans reach the same temperature, which will be in between their starting temperatures.
b. Before the pans touch, the bottom pan is cooler than the top pan. Once the pans are touching, the top pan will transfer kinetic energy to the molecules in the cooler bottom pan until both pans reach the same temperature, which will be in between their starting temperatures.
a. Before the pans touch, the bottom pan is hotter than the top pan. Once the pans are touching, the bottom pan will transfer kinetic energy to the molecules in the cooler top pan until both pans reach the same temperature, which will be in between their starting temperatures.
Flori has one bucket of paint outside and one bucket of paint in her garage. Both paint buckets are the same size and have the same number of molecules. She brings the bucket in from outside and is going to stack it on top of the bucket in the garage.
How does the temperature of the garage bucket compare with the temperature of the outside bucket before the buckets touch? What will happen after the buckets have been touching for a while?
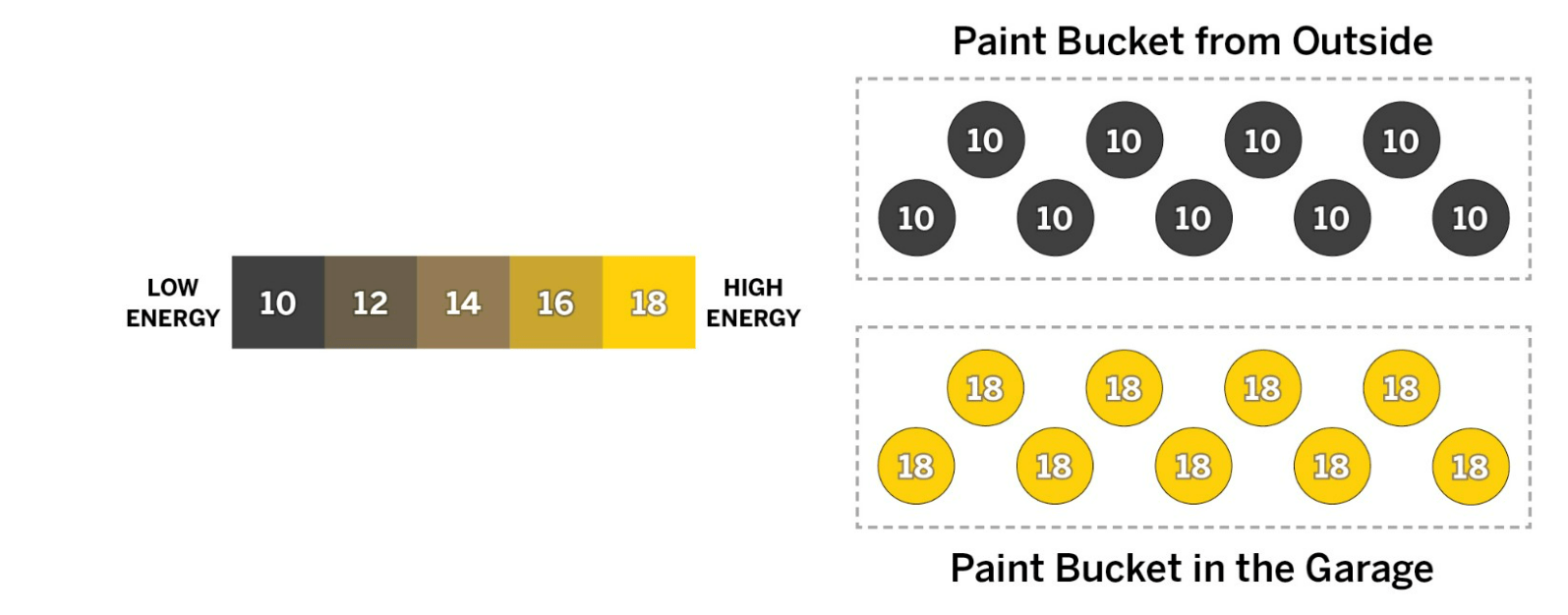
a. Before the buckets touch, the garage bucket is hotter than the outside bucket. Once the buckets are touching, the garage bucket will transfer kinetic energy to the molecules in the cooler outside bucket until both buckets reach the same temperature, which will be in between their starting temperatures.
b. Before the buckets touch, the two buckets are different temperatures. Once the buckets are touching, both kinetic energy and cold energy will transfer between the molecules of the two buckets until both buckets reach the same temperature, which will be in between their starting temperatures
a. Before the buckets touch, the garage bucket is hotter than the outside bucket. Once the buckets are touching, the garage bucket will transfer kinetic energy to the molecules in the cooler outside bucket until both buckets reach the same temperature, which will be in between their starting temperatures.
Energy cannot be created or destroyed, it can only be _______.
transferred
This chocolate brand’s slogan is “Melts in your mouth, not in your hands.”
What is M&M’s?