What type of reaction is this?
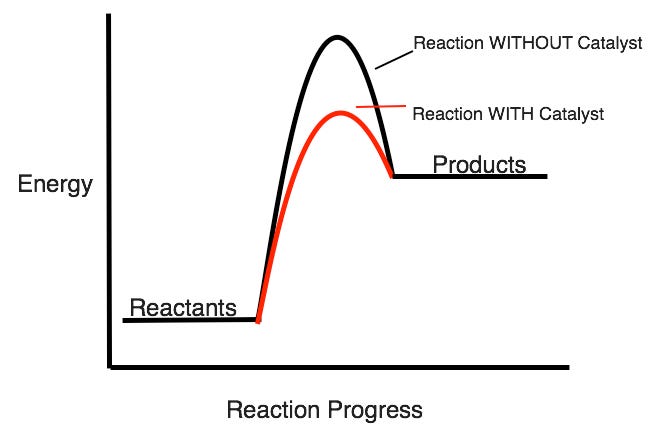
Endothermic
:max_bytes(150000):strip_icc():format(webp)/atom-57e1bb583df78c9cce33a106.jpg) The smallest unit of matter
The smallest unit of matter
Atom
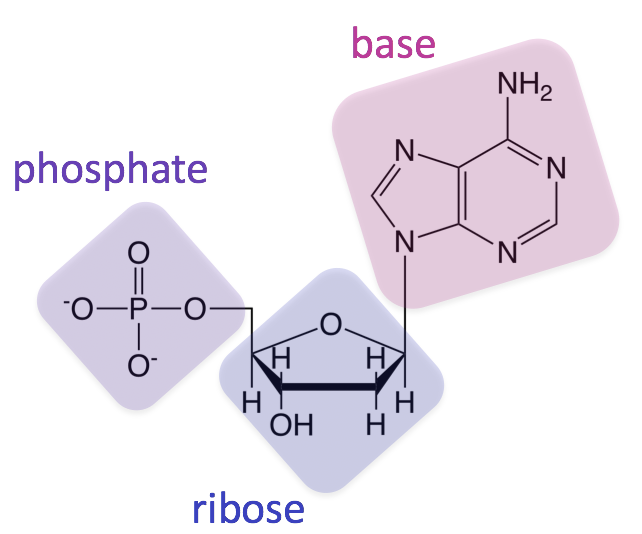 Which molecule is made up of Carbon, Hydrogen, Oxygen, Nitrogen, and Phosphorus?
Which molecule is made up of Carbon, Hydrogen, Oxygen, Nitrogen, and Phosphorus?
Nucleic Acid
 This type of bond forms when one electron is transferred to another atom
This type of bond forms when one electron is transferred to another atom
Ionic Bond
What is represented by letter A?
Substrate
:max_bytes(150000):strip_icc():format(webp)/atom-57e1bb583df78c9cce33a106.jpg) How many electrons would a neutral atom with 11 protons have?
How many electrons would a neutral atom with 11 protons have?
11 electrons
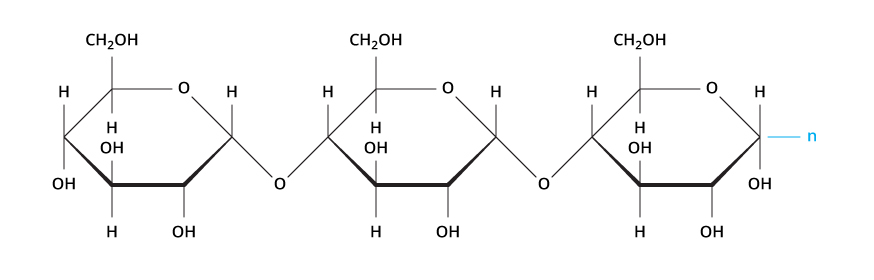 What are two functions of a carbohydrate?
What are two functions of a carbohydrate?
Main energy, structure,
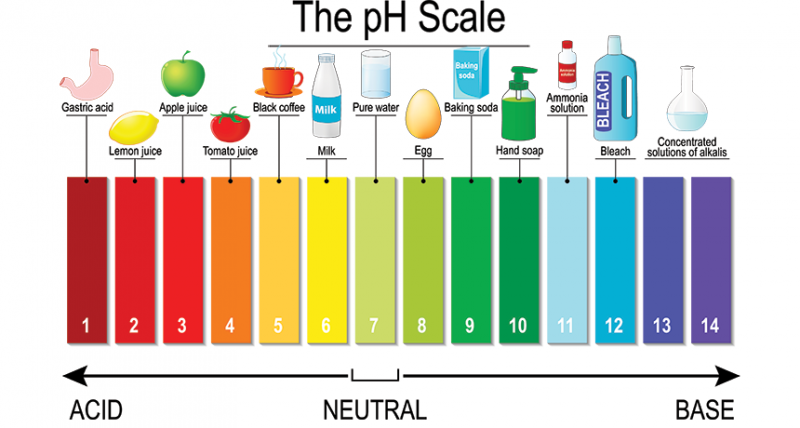 ________ releases H+ when it dissolves in water.
________ releases H+ when it dissolves in water.
Acid
What is represented by letter B?
Enzyme
:max_bytes(150000):strip_icc():format(webp)/atom-57e1bb583df78c9cce33a106.jpg) An Isotope is an atom that has a differing number of...
An Isotope is an atom that has a differing number of...
neutrons
Monomer of a protein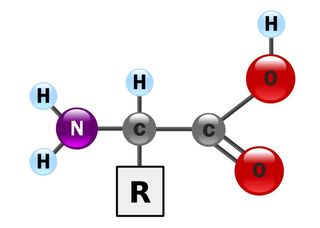
Amino Acid
Water is a ______ molecule. This means it has a partial negative and positive charge.
Polar
What is the area called where the enzyme and substrate come together (letter C)?
Active Site
 Hydrogen and oxygen are bonded together within ONE water molecule. What type of bond is this?
Hydrogen and oxygen are bonded together within ONE water molecule. What type of bond is this?
Polar Covalent
Enzymes are made of this macromolecule
Proteins
In water molecules, the bond between the partial positive charge of a hydrogen and the partial negative charge on a nearby oxygen is what type of bond?
Hydrogen Bond

Product
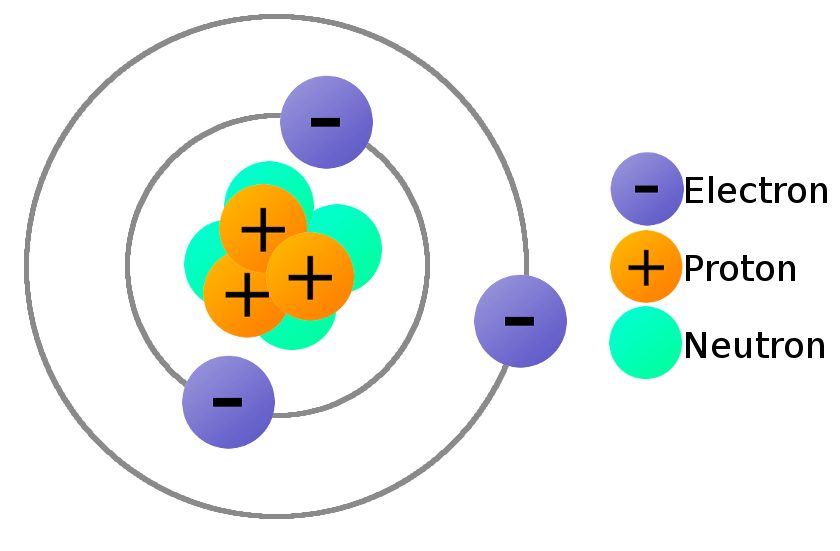 If an atom has 6 electrons, how many electrons will be in the outer shell?
If an atom has 6 electrons, how many electrons will be in the outer shell?
4
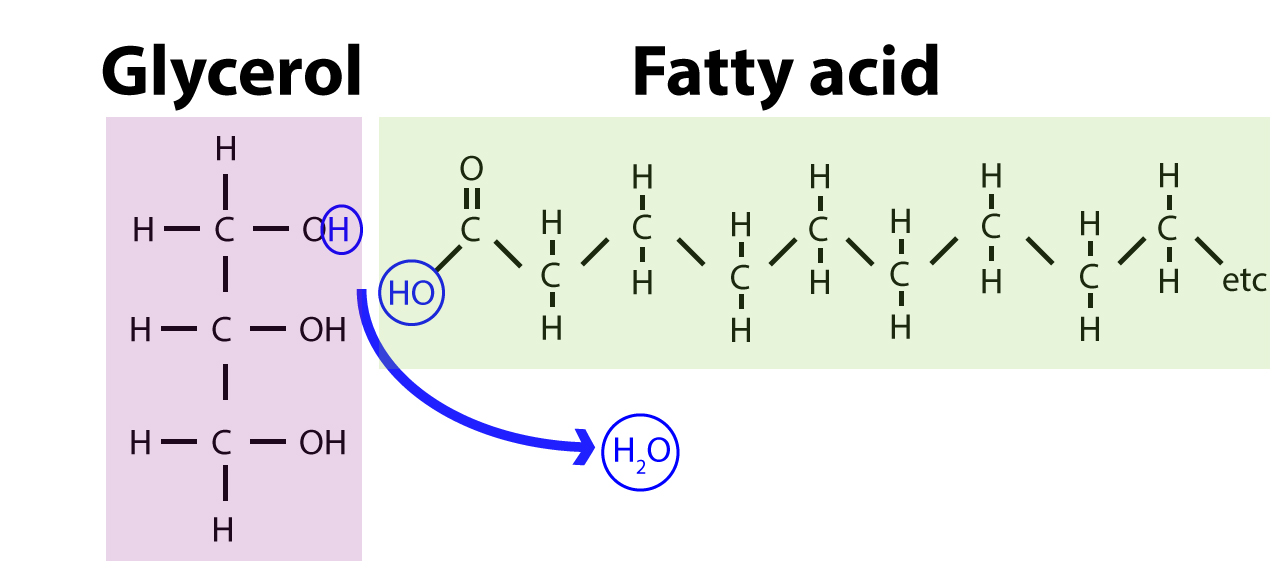 This macromolecule is nonpolar and found in membranes?
This macromolecule is nonpolar and found in membranes?
Lipids
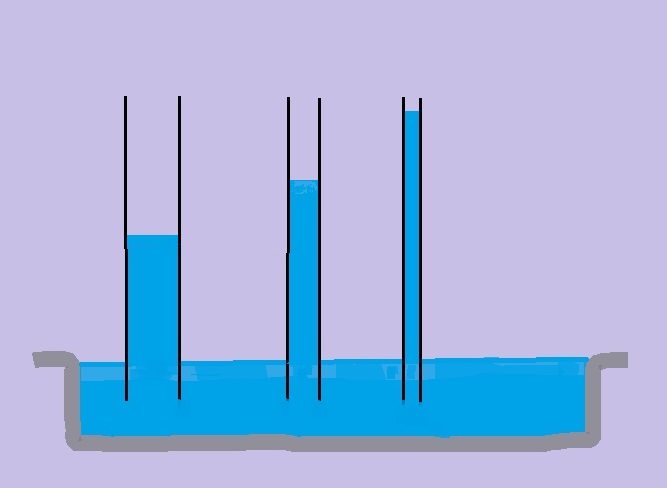 Water moving up in straw or glass tube against gravity describes what property of water? (Cohesion and Adhesion)
Water moving up in straw or glass tube against gravity describes what property of water? (Cohesion and Adhesion)
Capillary Action