Positively charged subatomic particle found in the nucleus an atom.
What is a proton?
Isotope name of an atom with:
6 protons
5 neutrons
What is Carbon-11?
What is 17 electrons?
What is the atomic number?
The mass number of an atom of Boron with:
5 protons
6 neutrons
3 electrons
What is 11amu?
An Ion that has a positive charge due to the loss of electrons.
What is a cation?
Isotope name of an atom with:
50 protons
54 neutrons
50 electrons
What is Tin-104?
The ion notation of an atom with:
31 protons
32 electrons
What is Ga-1?
The outermost energy level/ring of an atom.
What is the valence shell?
The name of the element that has a atom with the electron configuration of 1s2 2s2 2p6 3s2 3p1. (Assume neutral charge)
What is Aluminum?
What is the mass number?
Isotope notation of a neutrally charged atom of Chlorine-36.
What is 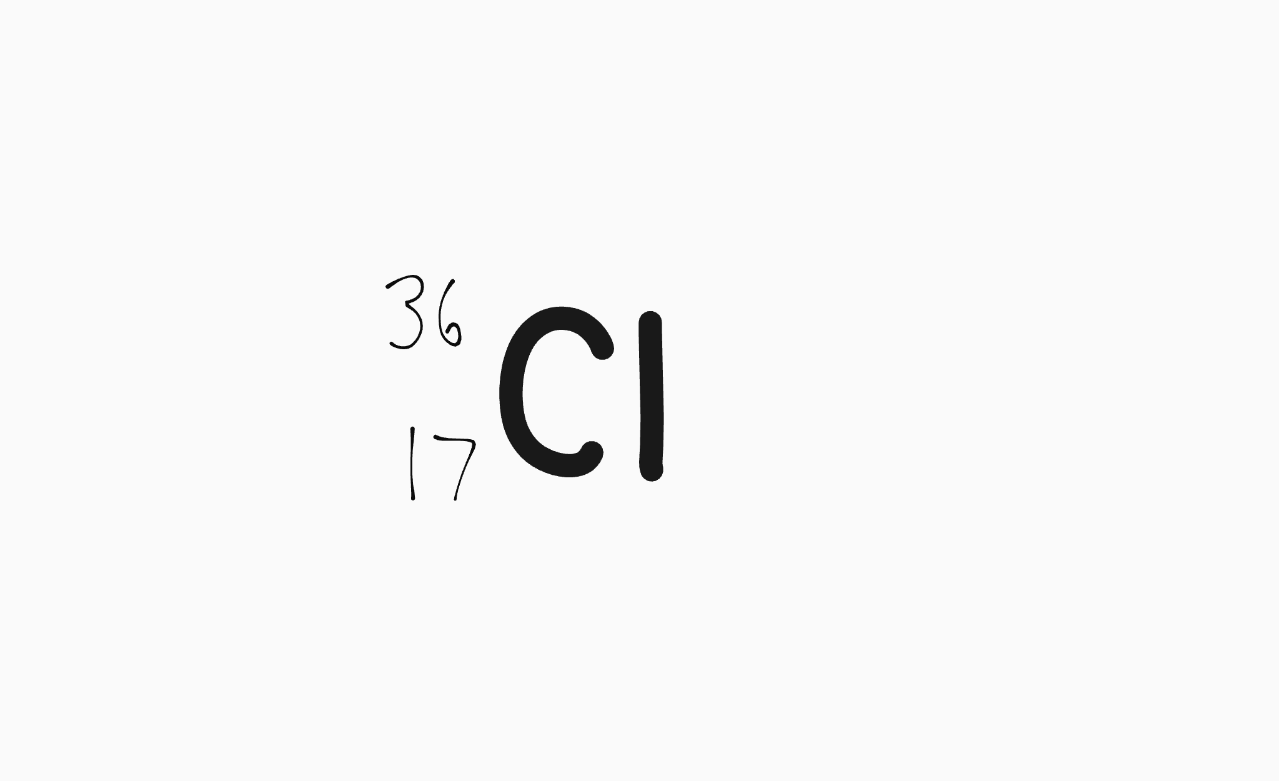
What is Mg-3?
What is electromagnetic force?
Average atomic mass of the Lithium isotopes:
Lithium-5 18% Abundance
Lithium-6 32% Abundance
Lithium-7 50% Abundance
What is 6.32amu?
Negatively charged subatomic particles in the outermost energy level, or ring, of an atom. The only particles that can be transferred between atoms.
What are valence electrons?
It's the number of neutrons in the isotopes Argon-38, Calcium-40, and Sulfur-36.
What is 20 neutrons?
The amount of electrons in each of the following ions: Li-2, C+1, F+4.
What is 5 electrons?
Weighted average of all the masses of the naturally occurring atoms of an element.
What is the average atomic mass?
The subatomic particles that reside in the nucleus of an atom.
What are protons and neutrons?
Atoms that vary in the number of neutrons; affects the mass of an atom.
What are isotopes?
The number of neutrons in this isotope: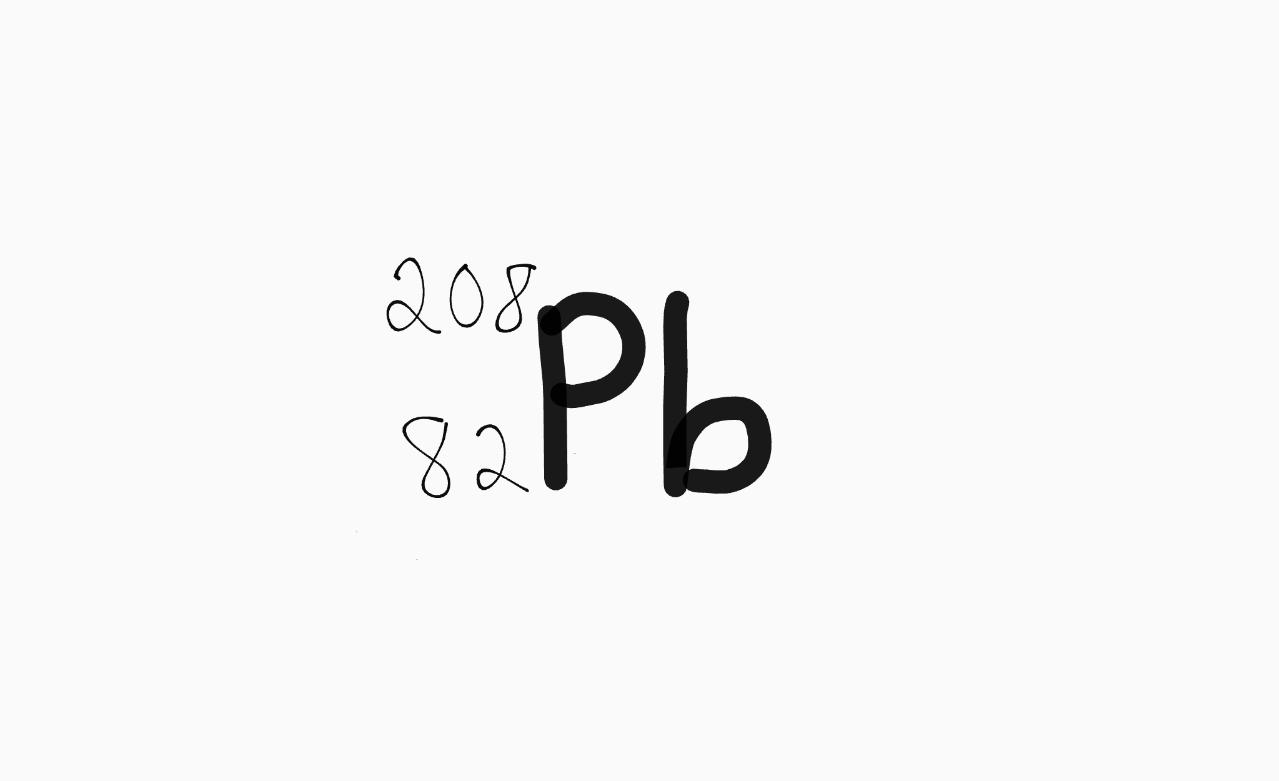
The total combined amount of subatomic particles in the following atom: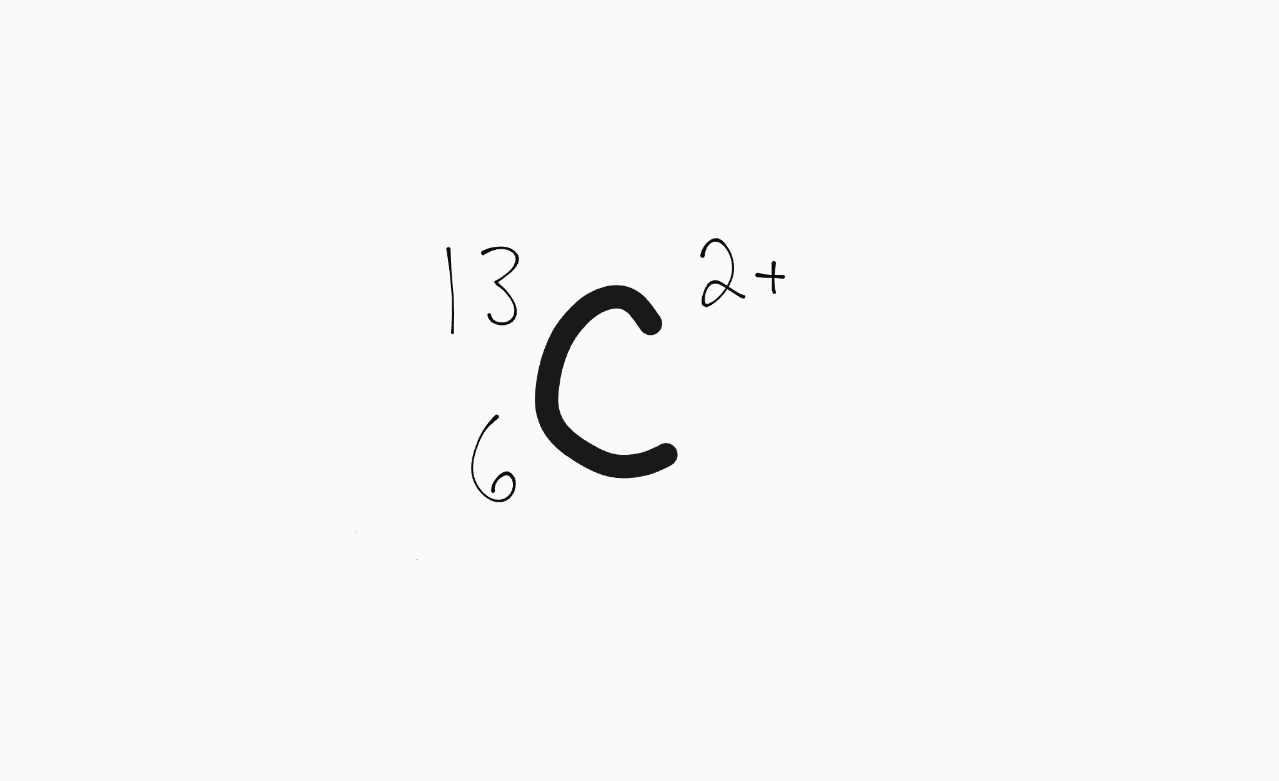
What is 17 subatomic particles?
(6p, 7n, 4e)
The arrangement of electrons in energy levels around the nucleus of an atom.
The number of valence electron in the atom shown in the electron configuration diagram:

What is 2 valence electrons?