The smallest particle of an element
What is an atom
Atomic Mass Unit
He was responsible for the gold foil experiment and he discovered the nucleus
Who is Rutherford
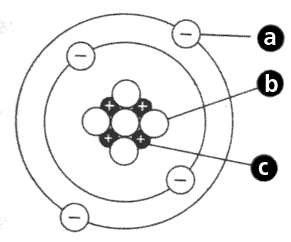 What is particle A?
What is particle A?
What is an electron
What is the element symbol for this element?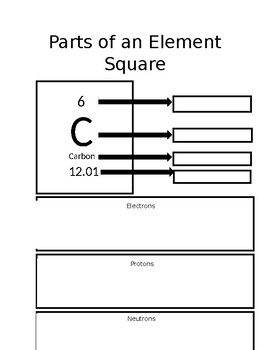
What is C
A representation of an object or system
What is a model
Meaning "indivisible"
What is atomos
He discovered the electron and used the plum pudding model
Who is Thomson
What is particle B?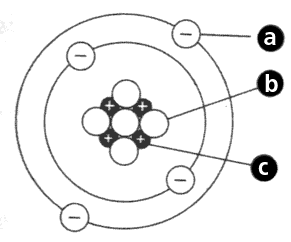
What is a neutron
What is the atomic mass for this element?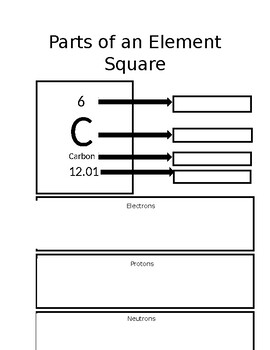
What is 12.01
Atoms of the same element that have different number of neutrons
What are isotopes
A region around the nucleus of an atom where electrons are likely to be found
What is electron cloud
He was a Greek philosopher who said that all matter is made of tiny particles called "atomos" or atoms
Who is Democrius
What is particle C?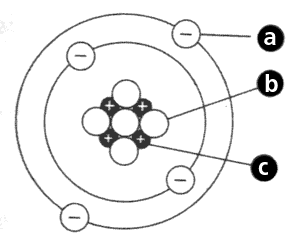
What is a proton
What is 6
Model of an atom that shows electrons in circular orbits around the nucleus
What is a Bohr Model
The total number of protons and neutrons in the nucleus of an atom
What is mass number
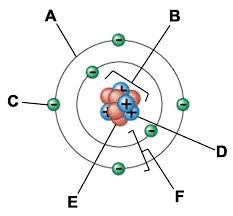
What is the name of part B?
What is the nucleus
Rows that go across from left to right are called: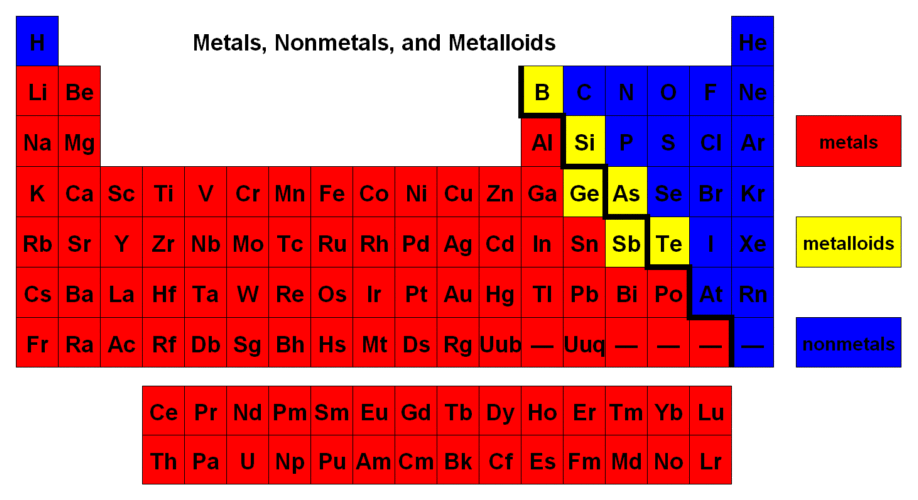
What are periods
A unifying explanation for a broad range of hypotheses and observations that have been supported by testing
What is a theory
The weighted average of the masses of the isotopes of an element
What is atomic mass
He was a British chemist who published his own atomic theory in 1803
Who is Dalton
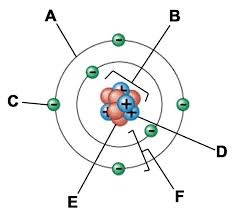
What is the name of part F?
What is electron cloud or energy level.
Columns that go up and down are called:
What are groups