Write the name of the following ionic compound: LiBr
Lithium bromide
Covalent bonds are not concerned with the charges of non-metals but the __________ is used for compounds and uses the same numbers.
combining capacity
What group(s) of the periodic table includes Halogens?
Group 17
Write the name for the following ionic compound: Ca(CO3)
Calcium carbonate
What group of the periodic table consists of the noble gases?
Draw a Lewis diagram for CO2
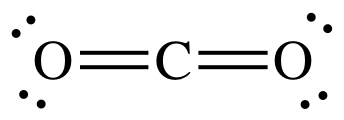
Write the name of the following formula: Mn3P2
Manganese (II) Phosphide
What is the name for electrons that are paired up and not shared with another atom?
lone pair
Draw a Lewis diagram for Phosphorus trifluoride

Write the name and formula for the following compound: Copper (II) nitrate

Write the name of the following formula: Ru2O3
Ruthernium (III) oxide
What was the covalent molecule Mr. Mac could not, for the life of him, model that had a 4- charge.
Silicon tetroxide
Draw a Lewis diagram for C2H2

(DAILY DOUBLE). When drawing a lewis dot diagram for CHCl3, Will included 3 chlorine atoms and one hydrogen atom, each with 6 valence electrons. At the centre, he had one carbon with 4 bonds. His total number of valence electrons were 32. What did he do wrong and how many valence electrons should he have
You cannot have 6 electrons on the terminal hydrogen which only wants 2. The correct number of valence electrons is 26.