An element consists of __________ kind of atom throughout.
one
A compound consists of ______________ kinds of atoms chemically bonded to each other.
two or more
In heterogeneous mixtures, particles are distributed or spread ________________. ( Evenly or Unevenly)
unevenly.
Chemical changes are ____________ (reversible, irreversible)
irreversible
________ is anything that takes up space and has mass.
Matter
What is the smallest unit of an element that maintains all the properties of that element?
Atoms
A compound differs from a mixture in that a compound always has a
a. Homogeneous composition
B. Heterogenous composition
c. Minimum of three components
D. maximum of three components
A.
True or false: heterogenous mixtures can be a mixture of solids, liquids, and/or gases.
True.
Mixtures can be any combination of all states of matter: solids, liquids, and gases.
True or false:
In a physical change, a new substance is created.
False.
Bonus: Rewrite the phrase so it is true.
Joshan did an experiment to differentiate between a homogenous mixture and a heterogeneous mixture. In beaker A he mixed 10g of salt to 50g of water and in beaker B he mixed 10g of flour to 50g of water. What is the independent variable in his experiment.
The solutes( flour and salt)
These are either all one element, or all one compound.
(2 words)
What are pure substances?
2Na + Cl2 ----> 2NaCl
The-______ sodium combines with the_______ chlorine to form the________ sodium chloride.
A. Compound; Compound; Element.
B. Element; Compound; Mixture
C. Element; Element; Compound.
D. Compound; Element; Mixture
C. Element, element , compound
Provide 2 examples of heterogeneous mixtures.
vegetable soup, oil and water, cereal, muddy water, salad etc
Phase changes are physical changes because the chemical substance does not change.
True!
Bonus: List three phase changes.
David added chorine to his swimming pool after cleaning it. What type of mixture did he create and what type of change took place
a homogenous mixture and a physical change
This describes how easily a substance can be pulled into a wire.
What is ductility?
Which picture represents an element and which one represents a compound.
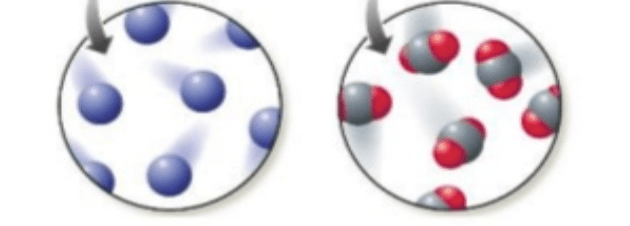
The picture on the left represents one element because it shows one kind of atoms. The picture on the right is a compound because it shows bonded atoms.
You observe that a sample of clear liquid has evaporated but left a powder like solid residue in the bottom of the container. This substance should be classified as a _________ ____________ or a ______________.
What is a homogenous mixture or a solution.
Fireworks are a classic example of chemical change/reaction.
What signs of chemical change elicit the most 'oooo's and 'ahhhhh's?
Unexpected colour change and release of heat/light.
Bonus! Is this reaction exothermic or endothermic?
This scientist was able to confirm that a golden crown was fraudulent by testing its density.
Eureka!
Who was Archimedes?
This property is measured in Kilograms.
What is mass?
Which of these statements are true about compounds? ( you may choose more than one)
A. Compounds can be found in the periodic table.
B. Compounds are pure substances.
C. Compounds are made up of two or more pure substances.
D. Compounds can be separated by physical means.
B and C
Compounds are pure substances and are made up of two or more elements.
*Elements can be found in the periodic table.
*Compounds can only be separated by chemical means.
What defines a mixture?
A. A mixture can be broken down into its basic substances through physical means.
B. A mixture cannot be broken down into its basic substances through physical means.
C. A mixture is created through a chemical reaction
D. A mixture is described by a chemical formula
A.
This is the proper term for a solid created by a reaction between two liquids.
Also, snowfall and rain.
What is a precipitate?
Pure substances have fixed melting and boiling point, which of the following are pure substances.
A. Both elements and compounds are pure substances
B. only elements are pure substances
C. neither elements nor compounds are pure substances
D. elements compounds and mixtures are pure substances
A. both elements and compounds are pure substnaces
How many atoms are represented in the formula CaCO3?
5
This is the chemical formula of salt.
What is sodium chloride, or NaCl?
Air is a mixture of about 78% nitrogen and 21% oxygen. Would air be a heterogeneous or homogeneous mixture? Why?
Homogenous mixture, the different gases cannot be distinguished
What is the formation of a gas?
With mixtures all _________ are mixtures, but not all _________ are _____________
All solutions are mixtures, but not all mixtures are solutions