What is the equation that explains the forces upon charged particles?
What is Coulomb's Law F=kqq/r2
What is the entirity of all forms of light called?
What is the Electromagnetic specrum?
The number of waves per time is called the ___________ and it is measured in a unit called ______________?
What is the frequency and Hertz?
1s2 2s2 2p5 is the electron configuration for what element?
What is flourine.
Draw the Lewis Dot diagram for Magnesium
Mg :
What do we call the forms of light from when an electron returns to the ground state from an excited state?
What is the emission spectrum?
The distance from the crest of one wave to the crest of another wave is called the_______?
What is the wavelength?
1s2 2s2 2p6 3s2 3p6 4s2 is the electron configuration for what element?
What is Calcium
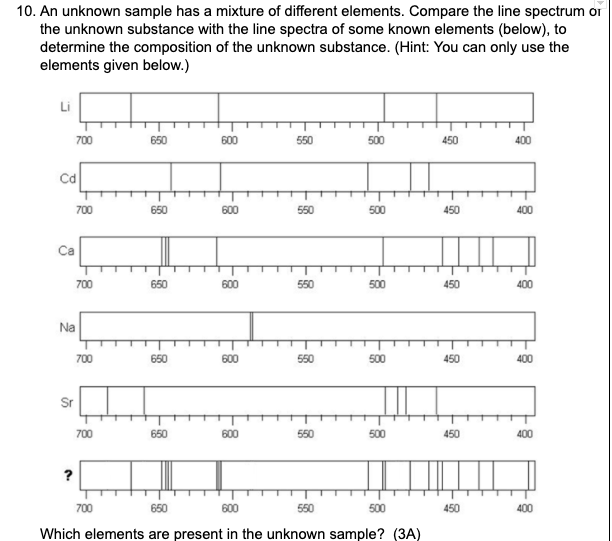
What are Lithium and Calcium?
What is the frequency of 556nm of light?
Use speed of light as 3.0E8 m/s
What is 5.40E14 Hertz?
f = speed of light / wavelength is meter
f = 3.0E8/5.56E-7m
What is the Noble Gas Notation for Chromium
What is [Ar]4s23d4
If a photon of light emits a red photon of light with a wavelength of 6.56E-7m (656nm) and a frequency of 4.56E14 Hertz? How much energy was released?
h=6.63E-34 Js
What is 3.03 E-19 Joules?