Covalent bonds form between___________ and ___________.
non-metal and a non-metal
Ionic bond form when electrons are ____________.
transferred
Bond formed from an overlapping "sea of electrons"?
What is a metallic bond?
These bonds produce poor conductors of heat and electricity.
What are covalent bonds?
A link between atoms resulting from the mutual attraction of their nuclei and electrons.
What is a chemical bond?
NaCl
What is ionic?
What are lone pairs?
Shows the type & number of atoms in a SINGLE molecule of a molecule compound.
What is the molecular formula?
Metals losing an electron form a ________, while non-metals gaining an electron form a __________.
What is a cation & an anion?
The ability of a substance to be drawn, pulled, or extruded through a small opening to produce a wire.
Ductility
These bonds produce crystals.
What are ionic bonds?
These are the three major types of chemical bonds.
What are Covalent, Ionic, and Metallic?
MgS
What is ionic?
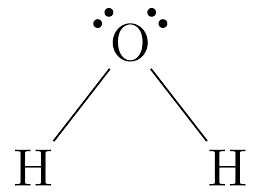
The shape of water.
A covalent bond produced by sharing three pairs of electrons.
What is a triple bond?
The energy released when one mole of an ionic crystal forms.
What is lattice energy?
Property that allows metal to be shaped into this...
What is malleability?
Refers to the uneven distribution of molecular charge.
What is polarity?
This increases as the ionic character increases.
What is the difference in electronegativities?
NaSO4
Both
The shape of ...
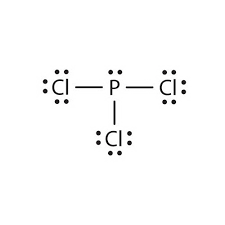
What is trigonal-pyramidal?
These use dots and dashes to represent the molecular compound like the following ......
What is a Lewis (dot) structure?
A group of covalently bonded atoms with a charge.
What are polyatomic ions?
This property of metals contributes to their characteristic of being a good conductor of heat and electricity.
What is the "sea of electrons" or free flowing electrons.
This refers to the bonding in molecules or ions that cannot be correctly represented by a single Lewis structure.
What is resonance?
These bonds have an uneven distribution of electrons.
What are Polar (covalent) bonds?
water
What is covalent?
The shape of ....
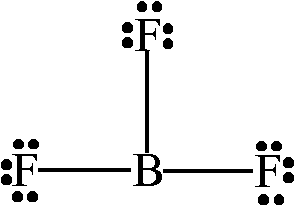
What is trigonal planar?
Draw Lewis structure for the following molecule. Show resonance structures, if they exist.
N2

Use electron dot structures to demonstrate the formation of the ionic compound involving
Na and S
See board
The strength of metallic bonds are measured with this.
What is heat of vaporization?
The type of notation seen here....
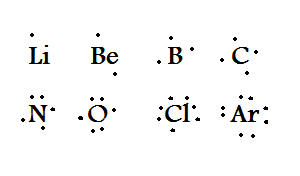
What is electron dot (notation)?
The force of attraction between polar molecules.
What are dipole dipole forces?
Sucrose
What is covalent?
This is the shape CH4
What is tetrahedral?
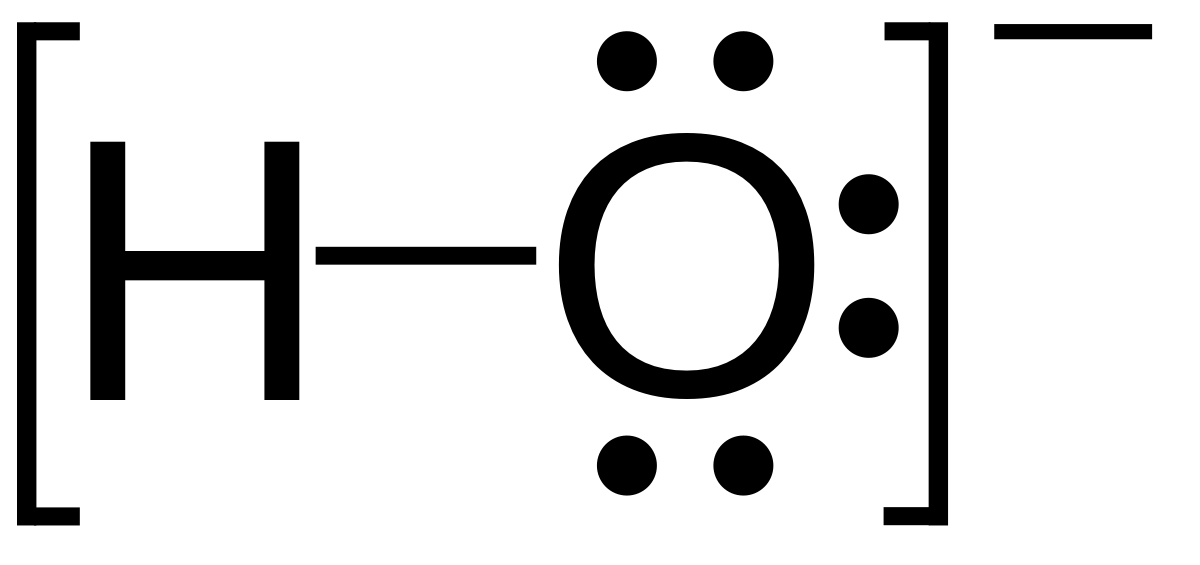
Draw Lewis structure for OH- ? Show resonance structures, if they exist.
What is the simplest collection of atoms from which an ionic compounds formula can be established?
Formula unit
The metals copper & zinc are bonded to make this section of the band....

What is brass?
Boron, phosphorus, & hydrogen all have this in common.
What are elements that violate the octet rule?
This is an instantaneous attraction that occurs because of the constant motion of electrons.
What are London Dispersion forces?
CFClH3
What is covalent?
This is what VSEPR stands for.
Valence Shell Electron Pair Repulsion
