A substance containing only one type of atom is called a(n):
What are the 3 properties of water?
cohesion, adhesion, and surface tension
A solution where water is the solvent.
Aqueous Solution
What would be considered the solvent and the solute in kool aid?
Solvent- water
solute- kool aide powder
Heat moves from ______ to _______.
hot to cold.
When a substance is neutral and does not change the color litmus paper, it has a pH level of _____.
seven
A compound is _______ type(s) of atom(s).
two or more
If a paperclip is able to float on water, that is an example of ________.
The part of the solution that does the dissolving.
Solvent
The term to describe how fast something dissolves.
Rate of dissolution
If a substance is cooled down, what will happen to the kinetic energy of the particles?
the particles will slow down.
Based on the picture, is the substance an acid or base?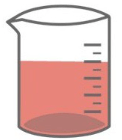
Acid
How many elements are in H2OCl3Na
4 Elements
When water molecules are attracted to one another, that is considered ___________.
cohesion
Creamer dissolving in coffee is an example of a _____.
Solution
To bother or to stir a solution.
Agitation
What is the kinetic energy (motion) of a solution if the temperature of a solution is hot?
High kinetic energy (fast motion)
True or False: Pure water is the ONLY neutral substance on the pH scale.
True!
What is the difference between an element and a compound?
Element is one type of atom and Compound is 2 or more types of atoms
adhesion
What is it called to form a solution by mixing evenly?
Dissolve
2g of sugar is placed into a glass of cold water and also into a glass of warm water. Which glass will have the fastest rate of dissolution?
Warm water
When does heat stop moving?
When it has reached equilibrium (when the temperature of both objects are the same.
If the pH is above 7, it is considered a _______.
basic
Identify the elements in the compound and how many atoms of each element.
C2H8Cl6O
Carbon-2
Hydrogen-8
Chlorine-6
Oxygen-1
How does cohesion help a water strider, "walk" on water?
the water molecules stick together under the bug, not allowing the water strider to break through.
Adding water to sweet tea in order to reduce the sweetness is an example of ____.
Dilution
What two factors affect the rate of dissolution?
Temperature and Surface Area
Agitation and Concentration
Explain what happens to the temperature and the kinetic particles if heat is applied to a flask overtime.
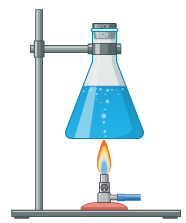
the temperature will rise and the particles will move faster.
Give at least 3 characteristics of an acid.
- has a pH lower than 7
- has a sour taste
- turns the litmus paper red
- has a sour taste
- corrosive
- can be found in both foods and nonfoods