Something that donates protons. Give an example
Bronsted-Lowry acid
examples: HCl, HBr, HI, etc.
How many carbons is in heptane? Is it saturated or unsaturated, so then how many hydrogens are in heptane?
7 carbons, saturated has 16 hydrogens
What is this structures name? Is it D or L?

ketopentose, L
What is the value for kilo?
1000
What is the name of CH3COOH
acetic acid, a weak acid
Balance this equation:
Fe2O3 + C --> Fe + CO2
2Fe2O3 + 3C --> 4Fe + 3CO2

3-methyl-1-hexanol
What are the four classifications of amino acids?
Nonpolar, polar, acidic (negatively charged), basic (positively charged)
What is the conversion of kg to g?
1000 g = 1 kg
What is the name of this structure. Is it primary or secondary

Butylamine, primary
Draw the lewis structure for NF3

Draw 5-chloro-2,3-dipropyl-7-octene
Will insert in email
What is the tertiary structure of a protein?
3D shape of the protein chain
Convert 340C to Fahrenheit
93.20 F
If [OH-]= 2.7 x 10-9 , what is [H+]? Based on [H+], is the solution acidic, neutral, or basic? Verify by calculating the pH
[H+] = 3.7 x 10-6 slightly acidic
pH= 5.43
What is the molecular geometry of PBr3
Trigonal planar
Draw hexanone and hexanal and hexanol. What functional groups are in each?
Hexanone: ketone

Hexanal: aldehyde

Hexanol: alcohol

What type of bond will be made? What disaccharide is formed from these two monosaccharides?
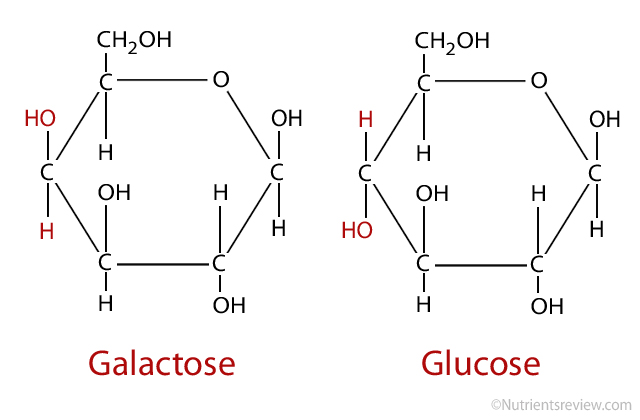
beta 1,4 - glycosidic bond will be made. Lactose is formed.
A sample of oxygen at room temperature occupies a volume of 250. L at 1.50 atm. What would be the volume of this gas at 7.50 atm at the same temperature?
50L
What type of bond is formed and what are we making? Is it a monosaccharide, disaccharide, or polysaccharide?

It is an alpha-1,4-glycosidic bond. We are making beta-maltose, a disaccharide.
Using this equation:
NO + O2 --> NO2 (is it balanced?)
If we have 0.765 moles of NO, how many grams of NO do we have? Using that number of grams, determine how many grams of NO2 we will have as well.
2NO + O2 --> 2NO2
22.96 g NO
35.20g NO2
Draw and name:
a molecule containing an ether
a molecule containing a thiol
Check for correct answer
This is D-glucose. From D-glucose, draw D-galactose. (hint, the only difference is on carbon 4). Oxidize D-galactose, showing it's structure. Reduce D-galactose, showing it's structure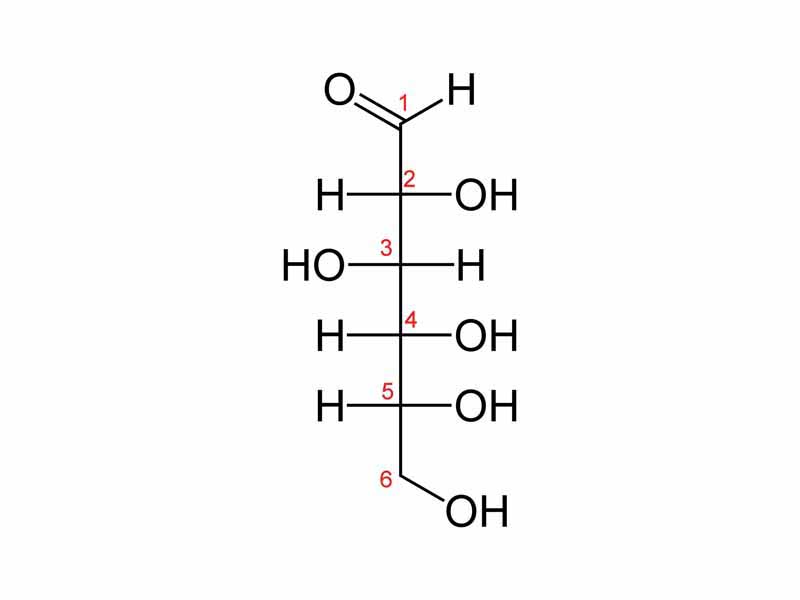 .
.
D-galactose:
When oxidized, aldehyde goes to a carboxylic acid. When reduced, aldehyde goes to an alcohol
If Kali had a cup of coffee with a density of 1.13g/mL and she drank out of a 0.43L cup, how many mg of coffee did she drink?
485,900 mg
Draw the reaction of the two reactants: ethyl ethanoate + KOH
Give names and structures of products.
Ethyl ethanoate:
+ KOH
-->
Product names: potassium ethanoate + ethanol
ethanol:
