Which of the following is an example of a pure substance? (select two)
-Homogenous Mixtures
-Heterogenous Mixtures
-Compounds
-Elements
Compunds & Elements

how many moles are there in 5.00 grams of NaCl (Sodium Chloride AKA table salt)?
7.64 X 10-2 mol NaCl
What are our three photon calculation equations?
E=hc/λ
c=λ*v
E=hv
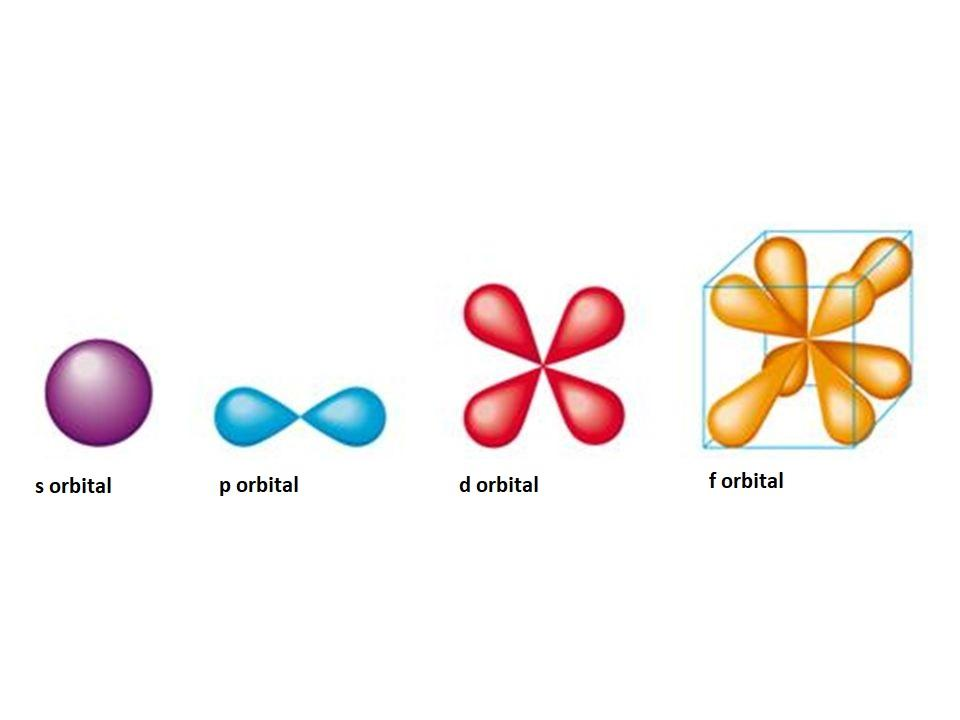
What are the four different potential shapes of orbitols? (Hint: the shapes are represented by letters)
Find the number of protons, neutrons, and electrons for Argon:

Atomic Number = Number of Protons: 18
NOT CHARGED; Number of Potons = electrons: 18
Mass # = Protons + Neutrons
OR
Nuetrons = Mass # - Protons: 22
You want to determine the density of honey. You measure the mass of your bowl as 7.23 oz. Then, you measure out 4.00 tbsp. of honey and add it to the bowl. Together their mass is 10.13 oz. What is the density of honey in ounces per tablespoon.
(These numbers are made up I can gauruntee that the answer we get is not the legit density of honey)
D=m/v
0.725 oz/tbsp
Express the appropriate units for the following variables:
1) E
2) v
3) λ
4) c
5) h
1) J
2) Hz, 1/s, s-1
3) m
4) m/s
5) J*s
What is the maximum number of electrons that an atom can contain in its f orbitol?
s: 1 orbital, 2 electrons.
p: 3 orbitals, 6 electrons.
d: 5 orbitals, 10 electrons.
f: 7 orbitals, 14 electrons.
Answer: 14 electrons max.
What are all the possible ways we could see the color green? (three possible ways)

A) Green is reflected back and all other colors are absorbed
B) Red is the only wavelength absorbed and all other colors are reflected. The orange and blue cancel out, the yellow and purple cancel out.
C) All colors are absorbed except for yellow and blue which mix to create green.
How many water molecules are there in 3.00 pounds of water?
How many hydrogen atoms are there in 3.00 pounds of water?
4.55 X 1025 H2O molecules.
9.10 X 1025 H atoms.
What is the frequency (v) in GHz, of a radio station with a broadcast wavelength (λ) of 783 cm?
v = 3.83 X 10-9 GHz
What element does the following electron configuration belong to:
[Ne] 3s23p3
P, "Phosphorus"
Where are the transition metals on the periodic table?
from columbs 3b to 2b
Convert 854 μL of water to tons of water.
(Hint: Use the density of water which can be found on your conversion sheet.)
9.41 X 10-7 tons of water.
Certain photons within the visible range have an energy of 4.94 X 10-22 kJ/Photon. What is the wavelength of one of these photons in nanometers? Given the wavelength, what is the color?
λ=402 μm
Color: Purple
Write the orbital diagram for Radon (Rn).
[Xe] |↑↓| |↑↓|↑↓|↑↓|↑↓|↑↓|↑↓|↑↓| |↑↓|↑↓|↑↓|↑↓|↑↓| 6s 4f 5d
|↑↓|↑↓|↑↓|
6p
What is the general pattern on the periodic table that describes the trend in size and trend in ionization energy for elements?
Down and to the left, atomic radius increases
Up and to the right, ionization energy increases
In a recent Grand Prix, the winner completed the race with an average speed of 229.8 km/h. What was the speed in miles per hour, meters per second, and feet per second?
142.8 mi/h; 63.8 m/s; 209 ft/s
The wavelength of a specific radio show is is 4183 pm. What is it's frequency in dH?
7.172 X 1017 dHz
Write the orbital diagram that means the same as the following electron configuration:
[Xe] 6s24f145d106p4
What element does this belong to?
[Xe] |↑↓| |↑↓|↑↓|↑↓|↑↓|↑↓|↑↓|↑↓| |↑↓|↑↓|↑↓|↑↓|↑↓| 6s 4f 5d
|↑↓|↑|↑|
6p
Element: Po, Polonium