The Rutherford Gold foil experiment, showed the existence of this atomic structure.
What is the nucleus?
The name of this illustrated atomic orbital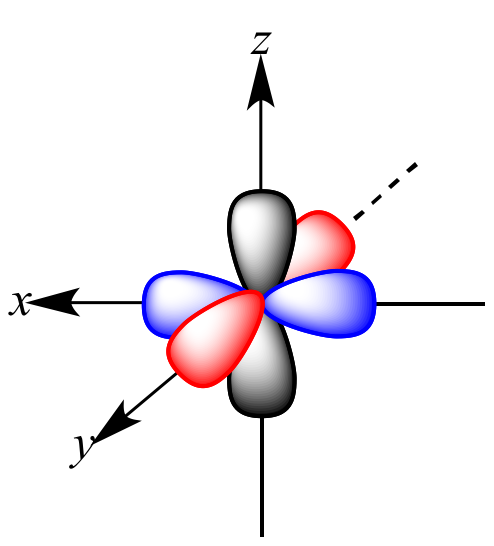
What is the p-orbital?
The number of orbitals occupied in a Helium (He) atom?
What is one orbital? (1s orbital)
What is the total number of neutrons in the below atom:
What is 34 neutrons? (63-29= 34 neutrons)
The number of valence electrons that atoms "tend to"
What is 8?
The number of electrons in an Al3+ atom

What is 10 electrons?
This quantum number gives you information about the angular orientation of an orbital.
What is the third quantum number (ml)?
The number of orbitals occupied in a Cu atom.

What is 7 orbitals? (1s, 2s, 2p, 3s, 3p, 4s, 3d)
How many total atoms in 1 mol of Cu?
What is

Elements in the 17th column of the periodic table (F, Cl, Br, I...) are known as this.
What are halogens?
The total number of electrons allowed in an f orbital
What is 14?
The shorthand notation of the electron configuration of F-

What is [He]2s22p6 ?
The frequency produced when a photon absorbs 3.313 *10-34 J of energy.
What is 0.5 Hz? (= 3.313 *10-34 / 6.626 * 10-34)
The formal charge of each oxygen CO32-
What is -1,-1,0?
The two most common isotopes for Chlorine (Cl)
What are Cl-35 and Cl-37?
The number of angular nodes and radial nodes that a 5d orbital has.
What is 2 angular and 2 radial nodes?
When Zirconium (Zr) loses an electron, it will be taken from this specific orbital.

What is 5s?
The correct radial probability distribution curve for the hydrogen atomic orbital with principal quantum number, n = 3 and azimuthal quantum number, l = 1 is:
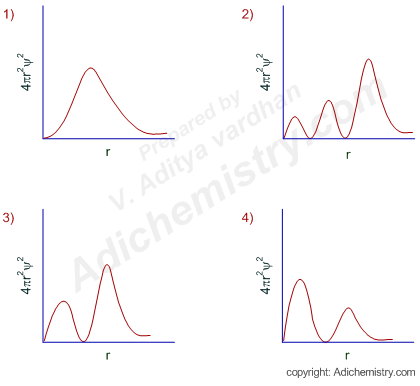
What is option 3?
(calculate number of radial nodes; 3-1-l = 3-1-1 = 1; so only one radial node. Both plots on bottom show that, but only plot 3 shows the maximum distribution occurring later on)
The number of resonance structures in CO32-
What is 3?
The year J.J. Thompson performed his cathode ray tube experiment that proved the existence of electrons
What is 1897?
The colors of a hydrogen atom emission spectrum.
What is violet, blue, green, and red?
The full electron spectrum of Copper (Cu).

What is 1s22s22p63s23p64s13d10 ?
Given the mass spectrum diagram below for Neon, what is the abundance (in %) of the Ne-22 isotope? (ignore diagram on right)
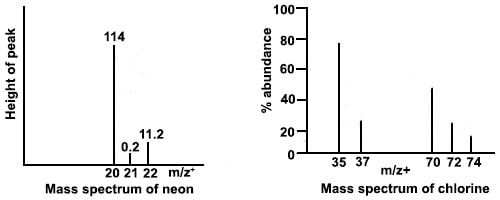
What is 8.93%? {(11.2/(11.2+0.2+114))*100}
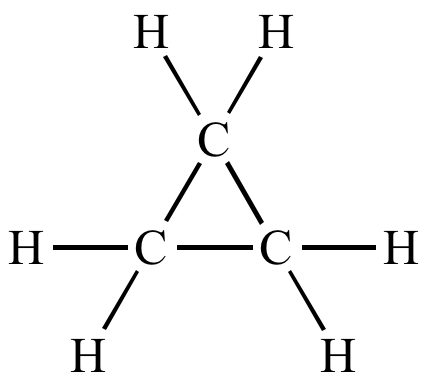
Draw the Lewis Dot Structure of C3H6 that contains NO DOUBLE BONDS.
What is