What is the identity of element X if the ion X-1 contains 16 electrons?
A) Sulfur
B) Silicon
C) Oxygen
D) Phosphorus
D) Phosphorus
Which of the following reactions are most likely to occur?
A) Mn + Mg- -> Mn- + Mg
B) Pb + Sn2+ -> Pb2+ + Sn
C) Zn + Cu2- -> Zn2-+ Cu
D) Au + Li+ -> Au+ + Li
C) Zn + Cu2- -> Zn2-+Cu
What is the electron configuration of Antimony(Sb)?
A) 1s22s22p63s23p64s24d104p65s25d105p3
B) 1s22s22p63s23p64s23d104p65s24d105p3
C) 1s22s22p63s23p64s23d104p65s24d105p6
D) [Ar]5s24d105p3
B) 1s22s22p63s23p64s23d104p65s24d105p3
How many Sigma and Pi bonds are in the molecule?
A) (σ): 15 (π): 4
B) (σ): 11 (π): 8
C) (σ): 25 (π): 4
D) (σ): 4 (π): 15
C) (σ): 25 (π): 4
What are the STP?
A) 1mol, 22.4L, 0*C, 1atm
B) 1mol, 11.0L, 0*C, 1mmHg
C) 1mol, 22.4L, 0*K, 1atm
D) 1mol, 11.0L, 0*K, 1mmHg
A) 1mol, 22.4L, 0*C, 1atm
Which molecule is the limiting reactant?
2LiF + Rb2S -> Li2S + 2RbF
If 40.0g LiF & 225.5gRb2S
A) LiF
B) Rb2S
C) Li2S
D) RbF
B) Rb2F
Which one of the following compounds is insoluble in water?
A) Li2CO3
B) CsCl
C) SO4Pb
D) BPO4
D) BPO4
Calculate the wavelength of a radio wave with a frequency of 32MHz. (1MHz=106 1/s)
(λ=c/v)
A) 0.11 m
B) 9.4 m
C) 1100m
D) 0.94 m
B) 9.4 m
Which element is the most Electronegative?
A) Cs
B) S
C) Al
D) Cl
D) Cl
Order the attraction forces from weakest to greatest
A) H-Bonding, Ion-DIpole, Dipole-Dipole, London Dispersion
B) London Dispersion, Dipole-Dipole, Ion-Dipole, H-Bonding
C) Dipole-Dipole, Ion-Dipole, London Dispersion, H-Bonding
D) London Dispersion, Dipole-Dipole, H-Bonding, Ion-Dipole
D) London Dispersion, Dipole-Dipole, H-Bonding, Ion-Dipole
If the percent yield for the following reaction is 47.3%, how many grams of Mg3N2 are needed to
produce 27.2 g of N2?
Mg3N2 -> 3Mg + N2
A) 207.2g
B) 46.4g
C) 414.3g
D) 185.9g
A) 207.2g
Calculate ΔH for the following reaction:
CH4(g) + 2O2(g) -> CO2(g) + 2H2O(g). ΔH=?
C(s) + O2(g) -> CO2(g). ΔH= -393.5kJ/mol
H2(g) +1/2O2 -> H2O(l). ΔH= -285.8kJ/mol
CH4(g) -> C(s) + 2H2(g). ΔH= +74.8kJ/mol
A) -754.1kJ.mol
B) -1039.9kJ/mol
C) -890.3kJ/mol
D) -604.5kJ/mol
C) -890.3kJ/mol
An electron cannot have the quantum numbers?
(n,l,ml)
A) 5, 3, 2
B) 2, 1, -2
C) 3, 2, -1
D) 3, 0, 0
B) 2, 1, -2
What is the hybridization of the right Carbon?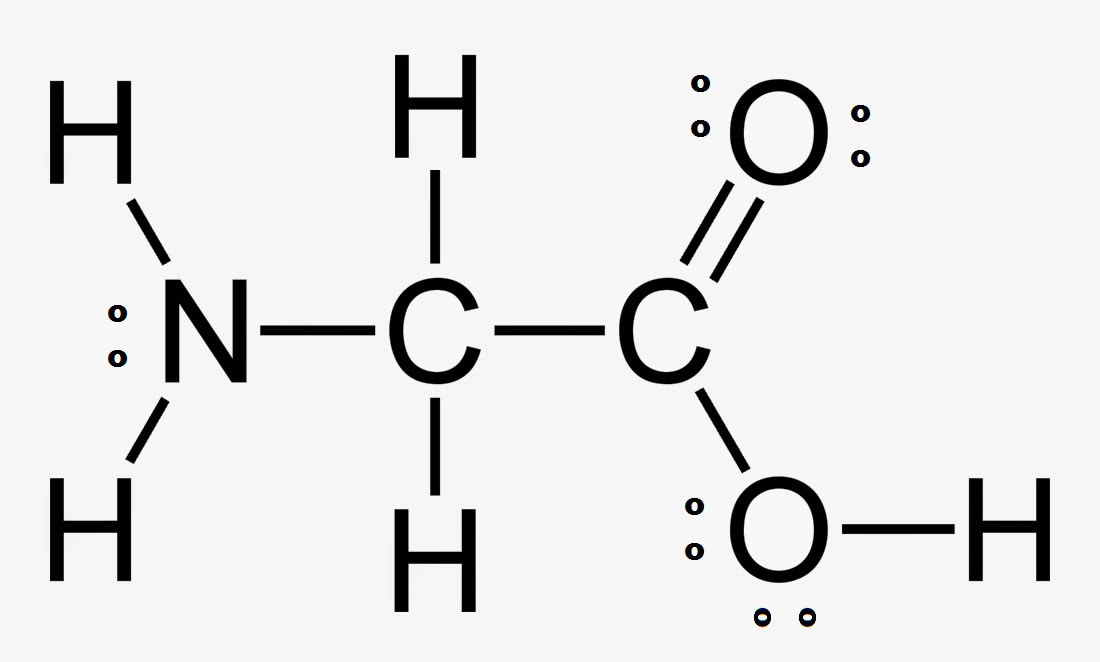
A) sp
B) sp2
C) sp3
D) sp3d
B) sp2
Which substance has the greatest Boiling point?
A) CH4
B) C2H6
C) C3H8
D) C6H12
D) C6H12
(Largest surface area)
Find the Empirical formula of: 23.02% Ba, 7.37% Al, 42.52% O, 27.09% Cl
A) Ba3Al5O48Cl14
B) Ba5Al4O50Cl10
C) Ba12Al7O56Cl27
D) Ba28Al9O52Cl33
A) Ba3Al5O48Cl14
A 22.5mL sample of an Acetic acid solution required 38.4mL of 1.719M NaOH for neutralization. What is the concentration of Acetic Acid?
A) 1.007M
B) 2.934M
C) 0.993M
D) 0.341M
B) 2.934M
Which of the following trends increase positively the further up and right on the periodic table?
(Select all that apply)
A) Electron Affinity
B) Ionization Energy
C) Electronegativity
D) Atomic Radius
B) Ionization Energy
C) Electronegativity
Using molecular orbital theory which species is Paramagnetic?(OFNe, BCN)
A) B2
B) C2
C) N2
D) O2
D) O2
Using Real Gas Laws what is the pressure of 20.0g O2 at 35.0*C and 18.0L?
Given that a= 1.360 L2atm/mol2 & b=0.0318L mol-1
(P+n2a/v2)(V-nb)=nRT
A) 0.877atm
B) 0.900atm
C) 1.753atm
D) 0.0982atm
A) 0.877atm
Bromine has an atomic mass of 79.904 amu. The Br-77 (76.832 amu) is 40.013%. What is the
amu of the other isotope?
A) 84.510 amu
B) 81.853 amu
C) 74.783 amu
D) 82.143 amu
B) 81.853 amu
A 1.625g sample of Benzoic Acid (MM=122.12g/mol) is burned in a bomb calorimeter. The heat capacity of the calorimeter is 7.93kJ/*C. During the combustion, the temperature of the calorimeter increases from 17.63*C to 42.67*C. What is the Molar heat of combustion on Benzoic Acid?
A) -14900 kJ/mol
B) +14900 kJ/mol
C) -237 kJ/mol
D) +237 kJ/mol
A) -14900 kJ/mol
(exothermic reaction is Molar heat is negative)
Determine the mass of an electron traveling at 4.25x109m/s with a wavelength of 3.44x10-4.
(λmv=h)
A) 4.53 x 10-40 Kg
B) 5.36 x 10-47 Kg
C) 8.19 x 10-21 Kg
D) 4.53 x 1028 Kg
A) 4.53 x 10-40 Kg
What is the Bond order of F2?
A) 1
B) 3
C) 4
D) 8
A) 1
Using the Phase diagram in which region might you find Superficial Fluid?
A) Region C
B) Region D
C) Region E
D) Region G
D) Region G