MnO4-
Permanganate
Give the net ionic equation for the following reaction
NaCl + AgNO3 --> NaNO3 + AgCl
Cl-(aq) + Ag+(aq) --> AgCl(s)
Give the condensed electron configuration for Ni
[Ar]4s23d8
What molecular geometry is CH4?
Tetrahedral
Give the periodic table trend for electronegativity
Increases as you go up a group and right across a period
Sulfate
SO4-2
Aluminum has a density of 2.7 g/cm3. If we have a 5.23 L sample, how many moles of Aluminum do we have?
523 mol
State the Pauli Exclusion Principle
No two electrons within an atom can have the same 4 quantum numbers
If a molecule has a central atom bonded to 3 atoms and no lone pairs, what is its molecular geometry and hybridization?
Trigonal planar
sp2
What is ionization energy?
The energy it takes to remove an electron from an atom
Ammonia
NH4+
If the heat of a reaction is 24.3kJ, the mass is 13.7g, and the temperature increased from 37 C to 54 C, what is the specific heat in terms of J/gK?
104.3 J/gK
Give the angular momentum quantum number (L) associated with a d orbital
L=2
Identify the hybridization for all of the carbons in this molecule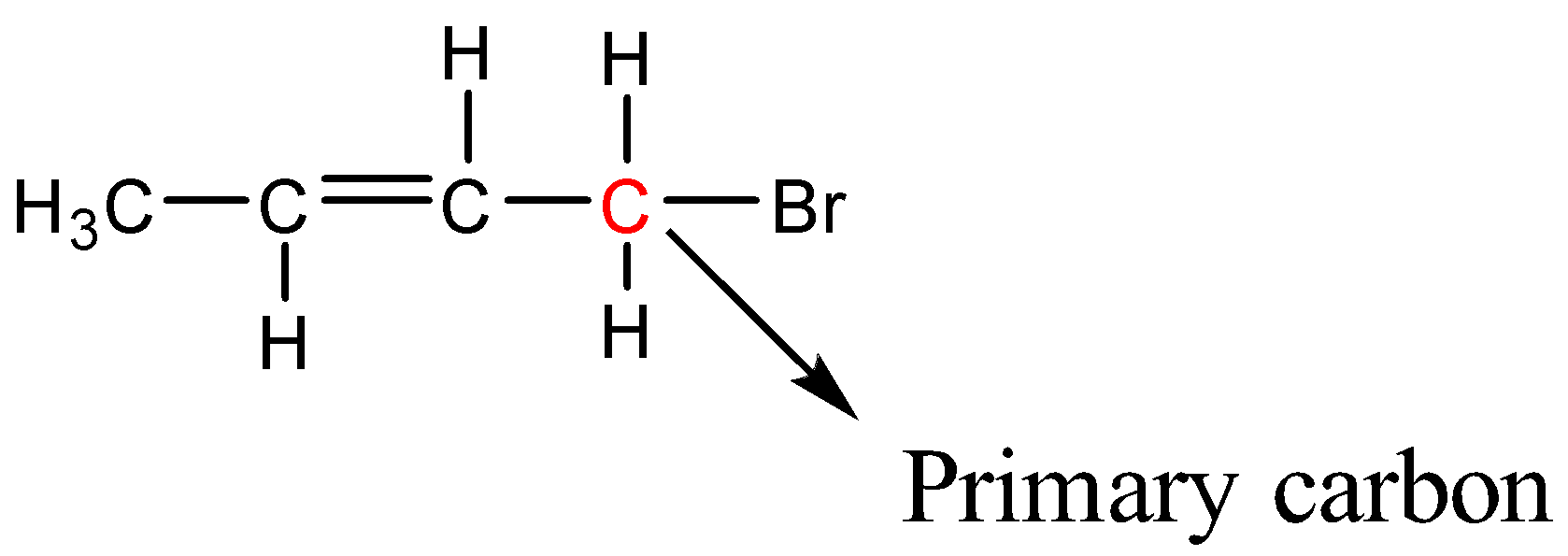
C1 and C4: sp3
C2 and C3: sp2
What is the trend for atomic radius?
Increases as you go down a group and as you go left across a period
CN-
Cyanide
NaOH + CaCl2 --> Ca(OH)2 + NaCl
If we have 40.0g of NaOH and 23.3g of CaCl2, what is the theoretical yield of Ca(OH)2?
If the actual yield is 27.24g, what is the percent yield?
15.70g
57.64%
Give the element that matches this the electron configuration [Kr]5s24d105p3
Sb (Antimony)
Draw a lewis structure for SF6. Give its molecular geometry and hybridization.
Octahedral
sp3d2
What is electron affinity, and what is its trend?
The ability for an atom to gain electrons. It increases as you go up a group and right across a period
ClO4-
Perchlorate
If the pressure is 784mmHg, the volume is 340.76mL, and the temperature is 24 C, what are the moles?
If the element is Silver, what is the mass in grams?
0.0144 moles
1.55 grams
List all of the L and ML values for n=3
L=2, ML=-2,-1,0,+1,+2
L=1, ML=-1,0,+1
L=0, ML=0
Trigonal pyramidal
sp3 hybridized
What is the electronegativity value for Oxygen, Bromine, and Fluorine?
3.5, 2.8, and 4.0