Significant figures in 0.00274003250
What is 9?
Protons in Uranium.
What is 92?
Formula mass of sodium chloride.
What is 58.44 amu?
Coefficient of C2H2 (acetylene) in the reaction for the combustion of acetylene.
What is 2?
10 mL of 2.0 M HCl is required to neutralize 15 mL of calcium hydroxide (Ca(OH)2). The concentration of the base is _______.
What is 0.67 M Ca(OH)2?
Kinetic energy associated with the random motion of atoms and molecules.
What is thermal energy?
No two electrons can have the same 4 quantum numbers.
What is the Pauli Exclusion Principle?
Shape of methane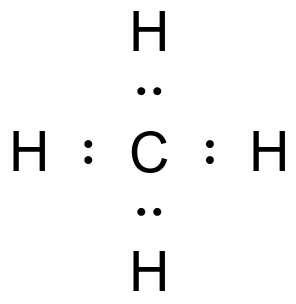
What is tetrahedral?
Unit for temperature when working with gases.
What is Kelvin?
1/1000 or 10-3
What does the prefix milli- mean?
Electrons in the nitride anion.
What is 8?
Moles of sodium hydroxide in 39.99 g.
What is one?
Precipitate formed when sodium chloride and silver nitrate are combined
What is silver chloride?
Volume of titrant solution required to react completely with the analyte.
What is the equivalence point?
The temperature of the cooling water as it leaves the hot engine of an automobile is 240oF. After it passes through the radiator it has a temperature of 175oF. 4 kg of water with a specific heat of 4.184 J/g will release ______ of energy to the surroundings.
What is 1.09 MJ? (1.09 x 103 kJ or 1.09 x 106 J)
l = 0 indicates a ____ orbital.
What is s?
Lone pairs on N in NH3.
What is 1?
The total pressure of a gaseous mixture is equal to the sum of the partial pressure of each individual gas.
What is Dalton's Law of Partial Pressure?
1000 or 103
What does the prefix kilo- mean?
Physicist credited with the nuclear model of the atom.
Who is Rutherford?
Moles of Cu2+ in 10.00 mL of 0.40 M CuSO4.
What is 4 x 10-3?
Oxidation number of sulfur in SO2.
What is +4?
0.4550 g of a mixture containing MgSO4 is dissolved in water and treated with excess Ba(NO3)2. The reaction produces 0.6168 g of BaSO4. The mass percent MgSO4 in the initial sample is
69.91%
Sign on change in enthalpy for an exothermic reaction.
What is negative?
Series of ions and atoms with identical electron configurations, usually a noble gas configuration.
What is isoelectronic?
Polarity of water
Polar
The volume of a gas increases as the number of moles of that gas increase.
What is Avogadro's Law?
Density of a 3.0 cm x 3.0 cm x 30.0 mm cube of Aluminum with a mass of 81.0 g
What is 2.7 g/cm3 or 2.7 g/mL?
Gives smallest whole number ratio of atoms or ions in a compound.
What is the empirical formula?
Grams of C6H12O6 in 100 g of a 1.8 percent by mass solution.
What is 1.8 g?
Moles of water produced when 0.5 moles of hydrogen react with excess oxygen.
What is 0.5 moles water.
Net ionic reaction for a strong acid with a strong base.
What is H+(aq) + OH-(aq) --> H2O(l)?
The oxidation of the sugar glucose, C6H12O6, is described by the following equation:
C6H12O6(s)+6O2(g)⟶6CO2(g)+6H2O(l) ΔH=−2816kJ
The metabolism of glucose gives the same products, although the glucose reacts with oxygen in a series of steps in the body. The metabolism of 1.0 g of glucose will produce how much energy?
What is 15.6 kJ?
An atom of gold contains _____electrons in the f orbitals.
What is 14?
Use a bond energy table to find the enthalpy of reaction for the combustion of ethane (C2H6)
What is -2831 kJ/mol?
Volume of 1 mole of a gas at STP
What is 22.4 L?
Mark McGuire hit 70 home runs in the 1998 season. There are 4 bases with 90 feet between each base. He ran _________ miles during that season just from home runs (1 mi = 5280 ft).
What is 5?
MnO2
What is manganese (IV) oxide?
A 750 mL of wine contains 15% ethanol (C2H5OH) by mass and has a density of 1.01 g/mL. This bottle contains _____ mol of ethanol.
What is 2.44?
10 grams of calcium react with 10 grams of chlorine to produce calcium chloride. The ______ is the limiting reagent.
What is chlorine?
Oxidation number on phosphorus in H2PO4-.
What is +5?
if a process can be represented as the sum of several steps, the enthalpy change of the process equals the sum of the enthalpy changes of the steps
What is Hess's Law?
The electron configuration for silver is an exception to the Aufbau rule, it is
1s22s22p63s23p64s23d104p65s14d10
The tendency of an atom in a molecule to attract the shared pair of electrons towards itself
What is electronegativity?
The volume of a gas is decreased 4-fold. The final pressure will be ______________ than the initial pressure.
4-fold greater.