True or False:
Boyles law states that if moles are temperature are constant the volume and pressure are proportional to each other, as temp rises so does pressure?
False
True/False: Consider having a 1.0 L sample of chlorine gas and a 1.0 L sample of fluorine gas at the same temperature and pressure. The molecules in both samples have the same average kinetic energy.
True/False: A 1.0 L sample of iodine gas at 1 atm will exert greater pressure than a 1.0 L sample of chlorine gas at 1 atm, if both are at the same temperature.
True
False
A mixture of 10 g of H2, 28 g of N2, and 3 moles of O2 is placed in a flask. When the partial pressure of nitrogen is 120 mmHg, what is the total pressure in the flask?
approx 1080mmHg
1.A gas absorbs 50 J of heat and performs 25 J of work by expanding. Calculate Delta E.
2.A system releases 100 J of heat and has 50 J of work done on it. Calculate Delta E.
1. 25J
2. −50J
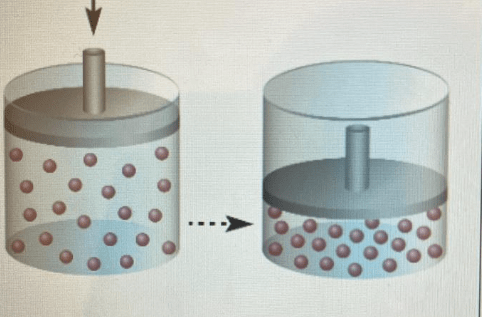
What statement regarding this picture is true?
A) the amount of moles have increased with constant pressure and temperature.
B) Pressure is doubled at a fixed volume and temperature.
C) The temperature has increased from 200K to 400K with a fixed amount of moles and pressure.
B
How many liters of oxygen are needed to combust 32 grams of methanol (CH3OH)? How many liters of carbon dioxide will be produced. The combustion takes place at STP.
34L of O2
22L of CO2
A gas mixture contains 2.50g He and 3.75g of Ne in a 3.50L container with a total pressure of 1.20atm. Calculate the mole fraction and partial pressure of each component in the gas mixture.
mole fraction of He 0.771
mole fraction of Ne 0.229
pressure of He 0.925atm
Pressure of Ne 0.275atm
Which Law would you use in order to find the final volume for the balloon?
A balloon is filled with 2.00 L of air at a temperature of 300 K. The balloon is then heated to 350 K. Assuming the pressure remains constant, what will the new volume of the balloon be?
Charles Law
A weather balloon is filled to a volume of 40.0 L at sea level, where the pressure is 760 mmHg and the temperature is 30.0°C. The balloon ascends to an altitude where the pressure drops to 350 mmHg and the temperature decreases to -20.0°C. What will be the new volume of the balloon at this altitude
7.25 L
A hydrocarbon with an empirical formula C3H4 takes 442 seconds to effuse through a plug. Under the same conditions of temperature and pressure, it takes 320 seconds for the same amount of nitrogen gas to effuse. What is the molar mass of the hydrocarbon and its molecular formula?
55.42g/mol
C9H12
A gas expands from an initial volume of 1.75 L to a final volume of 3.50 L against an external pressure of 1.20 atm. During the expansion, the gas absorbs 150 J of heat. What is the change in internal energy of the gas
−62.78J
Which of the following statements about gases is true?
(Select all that apply)
A) Gases do not have a definite shape or volume and will expand to fill their container.
B) The pressure of a gas decreases when the temperature decreases, if the volume remains constant.
C) Gases are highly compressible.
D) The molecules in a gas move faster as the temperature increases.
ALL are correct
Will 8.0 moles of chlorine gas in a 4.0 L tank at 27 o C act ideally? Van der Waals constants a=6.579 L2 • atm/mol 2 and b=0.05622 L/mol for chlorine gas.
No Cl2 does not act ideally
A student prepares a sample of hydrogen gas by electrolyzing water at 25C. She collects 315 mL of
hydrogen and oxygen at a total pressure of 555 mmHg. If the partial pressure of oxygen is 177 mmHg, calculate the partial pressure of hydrogen and the number of moles of hydrogen collected. What is the mole fraction of each of the gases in the vessel? The vapor pressure of water at 25C is 23.76 mmHg
Pressure of H2 - 354.mmHg
6.00E-3 mole H2
mole fraction H2 - 0.638
mole fraction O2 - 0.319
mole fraction H2O - 0.0428
A cylinder of volume 1.55L contains 2.00mol of Argon gas at room temperature. Which process does more work on the surroundings?
A. allowing the gas to expand isothermally to 3.25L against a constant pressure of 1.00atm.
B. allow it to expand reversibly and isothermally to 3.25L
A. -172J
B. -3670J
Process B does more work on the surroundings