The alkali metal in with 19 protons
What is Potassium?
0.0009455 in scientific notation
What is 9.455 x 10-4
0.0035 kilometers in meters
What is 3.5 meters?
This pure substance is formed when two or more different elements chemically combine in fixed ratios
What is a compound?
These components of a chemical equation appear to the left of the arrow
What are reactants?
The alkaline earth metal in the same period as xenon
What is strontium?
The number of significant figures in 0.0277
What is 3?
This is the constant used to convert from moles to atoms, molecules, or formula units.
What is Avogadro's Number?
NA=6.022x1023 atoms/mol
These compounds are comprised of cations and anions held together by weak electrostatic forces
What are ionic coumpounds?
These starting materials in a reaction are completely consumed and dictate how much product is made.
What is a limiting reactant?
The number of neutron given this nuclide symbol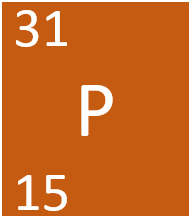
What is 16?
This is the molecular formula for a compound with a molecular weight of 56.06 g/mol and an empirical formula of CH2
What is C4H8?
This is the number of minutes per year
What is 525,600 minutes?
This pure substance consists of molecules held together by covalent bonds.
What is a molecular compound?
__ Ca + __N2 ---> ___Ca3N2
What is 3Ca + 1N2 ---> 1Ca3N2
This quantity on the periodic table indicates the number of protons in an element
What is Atomic Number?
This is the percent composition by mass of carbon in glucose C6H12O6
What is 40.0%?
The number of moles in 30.03 grams of carbon
What is 2.500 moles?
This is the name for SF6
What is sulfur hexafluoride?
This is the % yield when 5.43 grams of product are made compared with the calculated (theoretical) amount of 8.10
What is 67.0% yield?
These naturally occurring isotopes yield this Average Atomic Mass
Isotopic Mass Percent Abundance
27.98 92.21%
28.98 4.70 %
29.97 3.09 %
What is 28.09?
This is the empirical formula for sucrose given the molecule is 42.10% C, 6.49% H and 51.41% O
What is C12H22O11?
This is the mass in grams of 1.46 x1022 molecules of SiO2
What is 1.45 grams?
The formula for the ionic compound chromium (III) oxide
What is Cr2O3?
This many grams of sulfur dioxide SO2 are formed when 35.0 grams of carbon disulfide CS2 react with 35.0 grams of oxygen.
CS2 + 3O2 ---> CO2 + 2SO2
What is 46.7 grams of SO2?